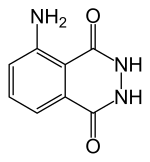
Luminol
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
5-Amino-2,3-dihydrophthalazine-1,4-dione
|
|
| Other names
5-Amino-2,3-dihydro-1,4-phthalazinedione
o-Aminophthaloyl hydrazide o-Aminophthalyl hydrazide 3-Aminophthalhydrazide 3-Aminophthalic hydrazide |
|
| Identifiers | |
|
521-31-3 |
|
| 3D model (Jmol) | Interactive image |
| ChEMBL |
ChEMBL442329 |
| ChemSpider |
10192 |
| ECHA InfoCard | 100.007.556 |
| EC Number | 208-309-4 |
| PubChem | 10638 |
|
|
|
|
| Properties | |
| C8H7N3O2 | |
| Molar mass | 177.16 g/mol |
| Melting point | 319 °C (606 °F; 592 K) |
| Hazards | |
| Safety data sheet | MSDS for luminol |
| NFPA 704 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Luminol (C8H7N3O2) is a chemical that exhibits chemiluminescence, with a blue glow, when mixed with an appropriate oxidizing agent. Luminol is a white-to-pale-yellow crystalline solid that is soluble in most polar organic solvents, but insoluble in water.
Forensic investigators use luminol to detect trace amounts of blood at crime scenes, as it reacts with the iron in haemoglobin. Biologists use it in cellular assays to detect copper, iron, and cyanides, as well as specific proteins by western blot.
When luminol is sprayed evenly across an area, trace amounts of an activating oxidant make the luminol emit a blue glow that can be seen in a darkened room. The glow only lasts about 30 seconds, but investigators can document the effect with a long-exposure photograph. Crime scene investigators must apply it evenly to avoid misleading results, as blood traces appear more concentrated in areas that receive more spray. The intensity of the glow does not indicate the amount of blood or other activator present, but only shows the distribution of trace amounts in the area.
Luminol may be synthesized by reverse-phosphorescence, two-step process. It begins from 3-nitrophthalic acid. First, hydrazine (N2H4) is heated with the 3-nitrophthalic acid in a high-boiling solvent such as triethylene glycol. An acyl substitution condensation reaction occurs, with loss of water, forming 3-nitrophthalhydrazide. Reduction of the nitro group to an amino group with sodium dithionite (Na2S2O4), via a transient hydroxylamine intermediate, produces luminol.
...
Wikipedia

