Lotronex
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Lotronex |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601230 |
| Pregnancy category |
|
| Routes of administration |
Oral (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50–60% |
| Protein binding | 82% |
| Metabolism | Hepatic (including CYP2C9, CYP3A4 and CYP1A2) |
| Biological half-life | 1.5–1.7 hours |
| Excretion | Renal 73%, faecal 24% |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
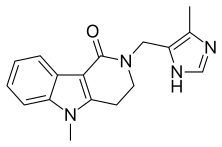
| Formula | C17H18N4O |
| Molar mass | 294.351 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Alosetron (original brand name: Lotronex LOW-trah-nex; originator: GSK) is a 5-HT3 antagonist used for the management of severe diarrhea-predominant irritable bowel syndrome (IBS) in women only. It is currently marketed by Prometheus Laboratories Inc. (San Diego). Alosetron was withdrawn from the market in 2000 owing to the occurrence of serious life-threatening gastrointestinal adverse effects, but was reintroduced in 2002 with availability and use restricted.
Alosetron was originally approved by the U.S. Food and Drug Administration (FDA) on February 9, 2000, after a seven-month review. At the time of the initial approval U.S. Food and Drug Administration (FDA) reviewers found that alosetron improved symptoms in 10% to 20% of patients.
Shipment to pharmacies started in March, 2000. On July 17, a health professional filed a report with the FDA on the death of a 50-year-old woman who suffered mesenteric ischemia. The report identified alosetron as the "primary suspect" in the death.
Alosetron was withdrawn from the market voluntarily by GlaxoWellcome on November 28, 2000 owing to the occurrence of serious life-threatening gastrointestinal adverse effects, including 5 deaths and additional bowel surgeries. The FDA said it had reports of 49 cases of ischemic colitis and 21 cases of "severe constipation" and that ten of the 70 patients underwent surgeries and 34 others were examined at hospitals and released without surgery. Through November 17, 2000, pharmacists had filled 474,115 prescriptions for alosetron. Severe adverse events continued to be reported, with a final total of 84 instances of ischaemic colitis, 113 of severe constipation, 143 admissions to hospital, and 7 deaths.
...
Wikipedia
