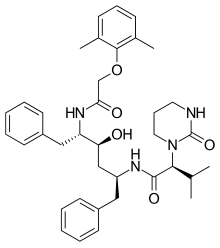
Lopinavir
 |
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a602015 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | 98-99% |
| Metabolism | Hepatic |
| Biological half-life | 5 to 6 hours |
| Excretion | Mostly fecal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C37H48N4O5 |
| Molar mass | 628.810 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Lopinavir (ABT-378) is an antiretroviral of the protease inhibitor class. It is used against HIV infections as a fixed-dose combination with another protease inhibitor, ritonavir, under the trade names Kaletra (high-income countries) and Aluvia (low-income countries). It was first approved by the FDA on 15 September 2000.
Lopinavir is highly bound to plasma proteins (98–99%).
Reports are contradictory regarding lopinavir penetration into the cerebrospinal fluid (CSF). Anecdotal reports state that lopinavir cannot be detected in the CSF; however, a study of paired CSF-plasma samples from 26 patients receiving lopinavir/ritonavir found lopinavir CSF levels above the IC50 in 77% of samples.
Side effects, interactions, and contraindications have only been evaluated in the drug combination lopinavir/ritonavir.
A 2014 study indicates that lopinavir is effective against the human papilloma virus (HPV). The study used the equivalent of one tablet twice a day applied topically to the cervices of women with high-grade and low-grade precancerous conditions. After three months of treatment, 82.6% of the women who had high-grade disease had normal cervical conditions, confirmed by smears and biopsies.
...
Wikipedia
