Ritonavir
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Norvir |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696029 |
| Pregnancy category |
|
| Routes of administration |
oral |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 98-99% |
| Metabolism | Hepatic |
| Biological half-life | 3-5 hours |
| Excretion | mostly fecal |
| Legal status | |
| Legal status | |
| ECHA InfoCard | 100.125.710 |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| Chemical and physical data | |
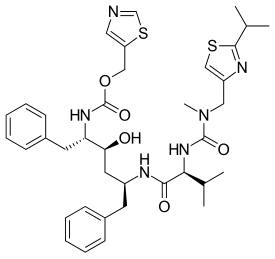
| Formula | C37H48N6O5S2 |
| Molar mass | 720.946 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Ritonavir, sold under the trade name Norvir, is an antiretroviral medication used along with other medications to treat HIV/AIDS This combination treatment is known as highly active antiretroviral therapy (HAART). Often a low dose is used with other protease inhibitors. It may also be used in combination with other medications for hepatitis C. It is taken by mouth. The capsules of the medication do not work the same as the tablets.
Common side effects include nausea, vomiting, loss of appetite, diarrhea, and numbness of the hands and feet. Serious side effects include liver problems, pancreatitis, allergic reactions, and arrythmias. Serious interactions may occur with a number of other medications including amiodarone and simvastatin. At low doses it is considered to be acceptable for use during pregnancy. Ritonavir is of the protease inhibitor class. It is often used to inhibit the enzyme that metabolizes other protease inhibitors. This inhibition leads to higher concentrations of these latter medication.
Ritonavir first came into use in 1996. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. Globally the wholesale cost in the developing world is between 0.07 and 2.20 USD per day. In the United States it costs about 9.20 to 55 USD per day depending on the dose.
Ritonavir is used along with other medications to treat HIV/AIDS.
When administered at doses effective for anti-HIV therapy, the side effects of ritonavir are those shown below. It is currently (2015) much more widely used at lower doses as a pharmacokinetic inhibitor. The adverse effects of these lower doses of ritonavir do not appear to have been extensively characterized.
...
Wikipedia
