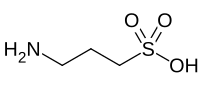
Homotaurine
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
3-Aminopropane-1-sulfonic acid
|
|
| Other names
Tramiprosate; Alzhemed; 3-APS
|
|
| Identifiers | |
|
3687-18-1 |
|
| 3D model (Jmol) | Interactive image |
| ChEMBL |
ChEMBL149082 |
| ChemSpider |
1584 |
| ECHA InfoCard | 100.020.889 |
| KEGG |
D06202 |
| PubChem | 1646 |
|
|
|
|
| Properties | |
| C3H9NO3S | |
| Molar mass | 139.17 g·mol−1 |
| Melting point | 293 °C (559 °F; 566 K) (decomposition) |
| Hazards | |
| R-phrases | R36/37/38 |
| S-phrases | S26 S36 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Homotaurine (3-amino-1-propanesulfonic acid (3-APS) or tramiprosate (INN)) is a synthetic organic compound. It is analogous to taurine, but with an extra carbon in its chain. It has GABAergic activity, apparently by mimicking GABA, which is it resembles.
Homotaurine was investigated in a Phase III clinical trial as a potential treatment for Alzheimer's disease that did not show efficacy in its primary endpoints. In preclinical studies it had been found to bind to soluble amyloid beta and inhibit the formation of neurotoxic aggregates. Homotaurine has also shown anticonvulsant activities, reduction in skeletal muscle tonus, and hypothermic activity.
Homotaurine has been reported as a GABA antagonist as well as a GABA agonist. In vitro studies have found that homotaurine is a GABAA partial agonist as well as a GABAB receptor partial agonist with low efficacy, becoming an antagonist and a displacing full agonist of GABA or baclofen at this receptor. In a study in rats, homotaurine reversed the catatonia induced by baclofen (the prototypical GABAB agonist), and was able to produce analgesia via the GABAB receptor, an effect that was abolished when CGP 35348, a GABAB receptor antagonist was applied.
One study in rats showed that homotaurine suppressed ethanol-stimulated dopamine release, as well as ethanol intake and preference in rats in a way similar to acamprosate. Acamprosate, the N-acetyl derivative of homotaurine, was approved by the FDA in 2004 to treat alcohol dependence.
...
Wikipedia
