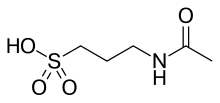
Acamprosate
 |
|
 |
|
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 11% |
| Protein binding | Negligible |
| Metabolism | Nil |
| Biological half-life | 20 to 33 hours |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.071.495 |
| Chemical and physical data | |
| Formula | C5H11NO4S |
| Molar mass | 181.211 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Acamprosate, sold under the brand name Campral, is a medication used along with counselling to treat alcohol dependence.
Acamprosate is thought to stabilize the chemical balance in the brain that would otherwise be disrupted by alcohol withdrawal. Studies find that acamprosate works to best advantage in combination with psychosocial support and can help facilitate reduced consumption as well as full abstinence.
Serious side effects include allergic reactions, abnormal heart rhythms, and low or high blood pressure, while less serious side effects include headaches, insomnia, and impotence. Diarrhea is the most common side-effect. Acamprosate should not be taken by people with kidney problems or allergies to the drug.
Until it became a generic in the United States, Campral was manufactured and marketed in the United States by Forest Laboratories, while Merck KGaA markets it outside the US. It is sold as 333 mg white and odorless tablets of acamprosate calcium, which is the equivalent of 300 mg of acamprosate.
Acamprosate is useful when used along with counselling in the treatment of alcohol dependence. Over three to twelve months it increases the number of people who do not drink at all and the number of days without alcohol. It appears to work as well as naltrexone.
Acamprosate is primarily removed by the kidneys and should not be given to people with severely impaired kidneys (creatinine clearance less than 30ml/min). A dose reduction is suggested in those with moderately impaired kidneys (creatinine clearance between 30ml/min and 50ml/min). It is also contraindicated in those who have a strong allergic reaction to acamprosate calcium or any of its components.
...
Wikipedia
