Hexol
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Tris[tetrammine-μ-dihydroxocobalt(III)]cobalt (III) ion
|
|
| Identifiers | |
| Properties | |
| Co4H42N12O18S3 | |
| Molar mass | 830.31 g·mol−1 |
| Sparingly soluble in water | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
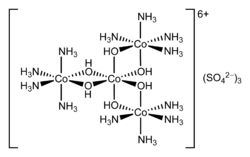
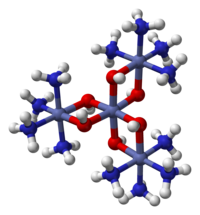
Hexol is the name for various salts of a coordination complex that has historical significance. The salts were the first synthetic non-carbon-containing chiral compounds. The sulfate salt has the formula {[Co(NH3)4(OH)2]3Co}(SO4)3.
Salts of hexol were first described by Jorgenson. The salt is prepared by heating [Co(NH3)4(H2O)2]3+ with dilute base such as ammonia followed by precipitation of the sulfate salt:
Depending on the conditions one obtains the 9-hydrate, the 6-hydrate, and the 4-hydrate. These salts exists as dark brownish-violet or black tabular crystals. The salts has low solubility in water. The cation can be quantitatively precipitated from its yellow-gray chromate and hexachloroplatinate salts. When treated with concentrated hydrochloric acid, hexol converts to cis-diaquotetramminecobalt(III) sulfate. In boiling dilute sulfuric acid, hexol degrades with evolution of oxygen and nitrogen.
In a historic set of experiments, Alfred Werner obtained chiral resolution by fractional crystallisation of the diastereomeric D-(+)-bromocamphorsulfonate salt. This ion has a high specific rotation of 2640°. More efficient methods involve the bis(tartrato)diantimonate(III) salt.
Werner also described a second achiral hexol (a minor byproduct from the production of Fremy's salt) that he incorrectly identified as a linear trimer. The second hexol is hexanuclear (contains six cobalt centres in each ion), not tetranuclear.
...
Wikipedia
