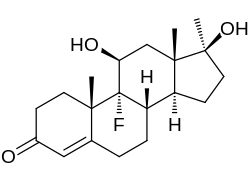
Fluoxymesterone
 |
|
| Clinical data | |
|---|---|
| Trade names | Halotestin, Ora-Testryl, Ultandren, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682690 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | G03BA01 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% Oral |
| Metabolism | Hepatic |
| Biological half-life | 9.5 hours |
| Excretion | urine |
| Identifiers | |
|
|
| Synonyms | Androfluorene; NSC-12165; 9α-Fluoro-11β-hydroxy-17α-methyltestosterone |
| CAS Number |
76-43-7 |
| PubChem (CID) | 6446 |
| IUPHAR/BPS | 2861 |
| DrugBank |
DB01185 |
| ChemSpider |
6205 |
| UNII |
9JU12S4YFY |
| KEGG |
D00327 |
| ChEBI |
CHEBI:5120 |
| ChEMBL |
CHEMBL1445 |
| ECHA InfoCard | 100.000.875 |
| Chemical and physical data | |
| Formula | C20H29FO3 |
| Molar mass | 336.441 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Fluoxymesterone, sold under the brand names Halotestin and Ultandren among others, is a synthetic, orally active androgenic-anabolic steroid (AAS) and a 17α-alkylated derivative of testosterone. While there are legitimate medical uses of fluoxymesterone, it is also abused, leading to the development of analytical techniques by which fluoxymesterone doping can be identified.
Fluoxymesterone is or has been used in the treatment of hypogonadism in males and breast cancer in women.
Fluoxymesterone has a relatively high ratio of androgenic to anabolic activity similarly to testosterone. Like many 17α-alkylated AAS, it has relatively low affinity for the androgen receptor (AR). However, its actions are mediated by the AR, most likely due to its relatively long elimination half-life of approximately 9.2 hours. It is approximately five times as potent as an AAS as testosterone. Unlike testosterone, fluoxymesterone has a 100% oral bioavailability, as the methylation of the C17α position of fluoxymesterone inhibits hepatic metabolism by enzymatic oxidation of 17β-hydroxyl, allowing its absorption into the bloodstream for transport around the body.
Fluoxymesterone has been found to act as a potent inhibitor of 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) (IC50 = 60–630 nM), with a potency comparable to that of the 11β-HSD2 inhibitor glycyrrhetinic acid. This unique action of fluoxymesterone is likely related to its 11β-hydroxyl group. 11β-HSD2 is responsible for the inactivation of the glucocorticoids cortisol and corticosterone (into cortisone and 11-dehydrocorticosterone, respectively). Inhibition of 11β-HSD2 by fluoxymesterone may result in mineralocorticoid receptor overactivation and associated side effects such as hypertension and fluid retention, and has been hypothesized to be involved in the cardiovascular and other adverse effects of fluoxymesterone.
...
Wikipedia
