Doxepin
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Sinequan, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682390 |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Oral, topical, intravenous, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 76% |
| Metabolism | Hepatic (CYP2D6, CYP2C19, CYP1A2,& CYP3A4 mediated) |
| Biological half-life | 8-24 hr (mean 17 hours); 31 hr for active metabolite, desmethyldoxepin |
| Excretion | Urine |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
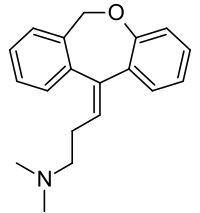
| Formula | C19H21NO |
| Molar mass | 279.376 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Doxepin is a tricyclic antidepressant marketed worldwide. It is marketed under many brand names worldwide.
Doxepin is used to treat depression, anxiety disorders, itchiness, trouble sleeping, and as a second-line treatment of chronic idiopathic urticaria (hives). Its oral formulations are FDA-approved for the treatment of depression, anxiety, and insomnia and its topical formulations are FDA-approved the short-term management (up to 8 days) of atopic dermatitis and lichen simplex chronicus. Whereas in Australia and the UK, the only licensed indication(s) is/are in the treatment of major depression and pruritus in eczema, respectively.
Its use in pregnant and lactating women is advised against, although the available preclinical (based on animal studies) evidence suggests it is unlikely to cause any deleterious effects on fetal development. The lack of evidence from human studies, however, means it is currently impossible to rule out any risk to the fetus and it is known to cross the placenta. Doxepin is secreted in breast milk and neonatal cases of respiratory depression in association with maternal doxepin use have been reported.
Known contraindications include:
Like other tricyclics (TCAs), doxepin is highly toxic in cases of overdose. Mild symptoms include drowsiness, stupor, blurred vision, and excessive dryness of mouth. More serious adverse effects include respiratory depression, hypotension, coma, convulsions, cardiac arrhythmia, and tachycardia. Urinary retention, decreased gastrointestinal motility (paralytic ileus), hyperthermia (or hypothermia), hypertension, dilated pupils, and hyperactive reflexes are other possible symptoms of doxepin overdose. Management of overdose is mostly supportive and symptomatic, and can include the administration of a gastric lavage so as to reduce absorption of the doxepin. Supportive measures to prevent respiratory aspiration is also advisable. Antiarrhythmic agents may be an appropriate measure to treat cardiac arrhythmias resulting from doxepin overdose. Slow intravenous administration of physostigmine may reverse some of the toxic effects of overdose such as anticholinergic effects.Haemodialysis is not recommended due to the high degree of protein binding with doxepin. ECG monitoring is recommended for several days after doxepin overdose due to the potential for cardiac conduction abnormalities.
...
Wikipedia
