Dimethylcarbamoyl chloride
 |
|
| Identifiers | |
|---|---|
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.001.099 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C3H6ClNO | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Dimethylcarbamoyl chloride is a reagent for transferring a dimethylcarbamoyl group to alcoholic or phenolic hydroxyl groups forming dimethyl carbamates, usually having pharmacological or pesticidal activities. Because of its high toxicity and its carcinogenic properties shown in animal experiments and presumably also in humans, dimethylcarbamoyl chloride can only be used under stringent safety precautions.
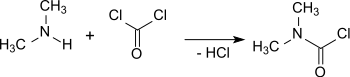
The production of dimethylcarbamoyl chloride from phosgene and dimethylamine (DMA) was reported as early as 1879 (reported as "Dimethylharnstoffchlorid" - dimethylurea chloride).
Dimethylcarbamoyl chloride can be produced in high yields (90%) at 275 °C by reacting phosgene with gaseous dimethylamine in a flow reactor. To suppress the formation of ureas excessive phosgene is used (in a 3:1 ratio).
The reaction can also be carried out at the laboratory scale with diphosgene or triphosgene and a aqueous dimethylamine solution in the two-phase system benzene+xylene/water in a stirred reactor with sodium hydroxide as an acid scavenger. However, considerably lower yields (56%) are achieved due to the hydrolysis sensitivity of dimethylcarbamoyl chloride .
Dimethylcarbamoyl chloride is also formed (together with methyl chloride) when reacting phosgene with trimethylamine.
...
Wikipedia