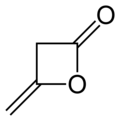
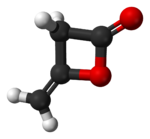
Diketene
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
4-methylideneoxetan-2-one
|
|||
| Other names
γ-methylenepropiolactone
|
|||
| Identifiers | |||
|
674-82-8 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider |
12140 |
||
| ECHA InfoCard | 100.010.562 | ||
|
|||
|
|||
| Properties | |||
| C4H4O2 | |||
| Molar mass | 84.08 g mol−1 | ||
| Density | 1.09 g cm−3 | ||
| Melting point | −7 °C (19 °F; 266 K) | ||
| Boiling point | 127 °C (261 °F; 400 K) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Diketene is an organic compound with the formula (CH2CO)2. It is formed by dimerization of ketene (IUPAC name: ethenone). Diketene is a member of the oxetane family. It is used as a reagent in organic chemistry. It is a colorless liquid.
Ketene is generated by dehydrating acetic acid at 700–750 °C in the presence of triethyl phosphate as a catalyst or (in Switzerland and the CIS) by the thermolysis of acetone at 600–700 °C in the presence of carbon disulfide as a catalyst.
The dimerization to diketene proceeds spontaneously at room temperature.
Heating or irradiation with UV light regenerates the ketene monomer:
Alkylated ketenes also dimerize with ease and form substituted diketenes.
Diketene readily hydrolyzes in water forming acetoacetic acid, its half-life in pure water is approximately 45 minutes a 25 °C at 2<pH<7.
Certain diketenes with two aliphatic chains, such as alkyl ketene dimers (AKDs), are used industrially to improve hydrophobicity in paper.
At one time acetic anhydride was prepared by the reaction of ketene with acetic acid:
Diketene also reacts with alcohols and amines to the corresponding acetoacetic acid derivatives. The process is sometimes called acetoacetylation. An example is the reaction with 2-aminoindane:
Diketene is an important industrial intermediate used for the production of acetoacetate esters and amides as well as substituted 1-phenyl-3-methylpyrazolones. The latter are used in the manufacture of dyestuffs and pigments. A typical reaction is:
...
Wikipedia