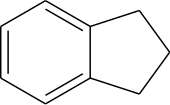
Indane
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
2,3-Dihydro-1H-indene
|
|
| Other names
Indane
Benzocyclopentane Hydrindene 2,3-Dihydroindene |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| 1904376 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.105 |
| 67817 | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C9H10 | |
| Molar mass | 118.176 g/mol |
| Appearance | Clear colorless liquid |
| Boiling point | 176.5 °C (349.7 °F; 449.6 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Indane or indan is a hydrocarbon petrochemical compound, with formula C9H10.
Derivatives include compounds such as 1-methyl-indane and 2-methyl-indane (where one methyl group is attached to the five carbon ring), 4-methyl-indane and 5-methyl-indane (where one methyl group is attached to the benzene ring), various dimethyl-indanes, and various pharmaceutical derivatives. Other derivatives can be obtained indirectly, e.g. the reaction of diethyl phthalate with ethyl acetate, using metallic sodium and ethanol as a catalyst. The reaction yields indanedione ethyl ester, which can react with the sodium ions yielding a salt. This can be reversed by adding an aqueous solution of hydrochloric acid.
Indane can also be converted in a catalytic reactor to other aromatics such as xylene.
Another derivative is 1,3-indandione.
Dr. David E. Nichols developed a family of indane derivatives that are empathogen-entactogens. They are very close derivatives of other empathogen-entactogens such as MDMA and MDA. However, they are not neurotoxic at therapeutic doses because the alpha-methyl group is bound and therefore cannot form a free radical. Examples include MDAI, MDMAI, and MDAT. Nichols also created an indane isomer of amphetamine, 2-aminoindane, and an iodized derivative 5-IAI.
Indane is also known to be used in the synthesis of sulofenur.
3. https://pubchem.ncbi.nlm.nih.gov/compound/10326#section=Top
...
Wikipedia
