Dicoronylene
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
benzo[10,11]phenanthro[2',3',4',5',6':4,5,6,7]chryseno[1,2,3-bc]coronene
|
|
| Other names
benzo[1,2,3-bc:4,5,6-b'c']dicoronene
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C48H20 | |
| Molar mass | 596.69 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
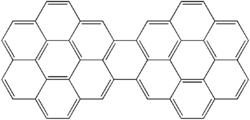
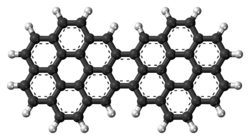
Dicoronylene is the trivial name for a very large polycyclic aromatic hydrocarbon. Its formal name is benzo[10,11]phenanthro[2',3',4',5',6':4,5,6,7]chryseno[1,2,3-bc]coronene (IUPAC name) or benzo[1,2,3-bc:4,5,6-b'c']dicoronene (name sometimes used in Chemical Abstracts). It has 15 rings and is a brick-red solid. Its formula is C
48H
20. Dicoronylene sublimes under high vacuum, 0.001 torr, between 250 °C and 300 °C.
Due to its large size and limited availability, the organic chemistry of dicoronylene is little known. Dicoronylene does undergo a Diels–Alder reaction with maleic anhydride on one or both of the central bay regions on either side of the bridging ring. The double bond of maleic anhydride forms two carbon–carbon bonds on the ends of the bay region, making a new six-membered ring. Heating removes the anhydride as carbon dioxide gas and gives the corresponding 16-ring and 17-ring PAHs.
Dicoronylene was first observed in the solid residue produced in coal gasification. This residue contained large amounts of coronene and ovalene. After these were extracted and identified, a reddish residue remained, which was sparingly soluble in organic solvents. Elemental analysis indicated that it was most likely the condensed dimer of coronene.
Dicoronylene was later discovered to occur as a by-product of the catalytic hydrocracking used in petroleum processing. It is formed when two coronene molecules fuse. It is estimated that catalytical hydrocracking produces several hundred metric tons of dicoronylene worldwide per year, making it the most prevalent large PAH. In this process, the analogous 18-ring PAH formed from coronene and ovalene (C
56H
22) is also formed in 1% to 20% proportions. It is purple in color.
...
Wikipedia
