Diels–Alder reaction
| Diels–Alder reaction | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Named after |
Otto Paul Hermann Diels Kurt Alder |
||||||||
| Reaction type | Cycloaddition | ||||||||
| Reaction | |||||||||
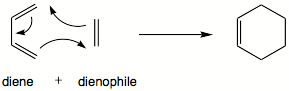
|
|||||||||
| Identifiers | |||||||||
| Organic Chemistry Portal | diels-alder-reaction | ||||||||
| RSC ontology ID |
RXNO:0000006 |
||||||||
The Diels–Alder reaction is an organic chemical reaction (specifically, a [4+2] cycloaddition) between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. It was first described by Otto Diels and Kurt Alder in 1928, for which work they were awarded the Nobel Prize in Chemistry in 1950. The Diels–Alder reaction is particularly useful in synthetic organic chemistry as a reliable method for forming 6-membered systems with good control over regio- and stereochemical properties. The underlying concept has also been applied to other π-systems, such as carbonyls and imines, to furnish the corresponding heterocycles, known as the hetero-Diels–Alder reaction. Diels–Alder reactions can be reversible under certain conditions; the reverse reaction is known as the retro-Diels–Alder reaction.
The reaction is an example of a concerted pericyclic reaction. It is believed to occur via a single, cyclic transition state, with no intermediates generated during the course of the reaction. As such, the Diels–Alder reaction is governed by orbital symmetry considerations: it is classified as a [4πS+2πS] cycloaddition, indicating that it proceeds through the suprafacial/suprafacial interaction of a 4π electron system (the diene structure) with a 2π electron system (the dienophile structure), an interaction that is thermally allowed as a 4n+2 cycloaddition.
A consideration of the reactants’ frontier molecular orbitals (FMO) makes plain why this is so. We note that for a ‘normal’ electron demand Diels–Alder reaction, the electron-rich diene’s Ψ2 is the highest occupied molecular orbital (HOMO) while the electron-deficient dienophile’s π* is the lowest unoccupied molecular orbital (LUMO). However, the HOMO-LUMO energy gap is such that the roles can be reversed by switching the substitution pattern: i.e. the diene’s Ψ3 might be considered the LUMO if electron withdrawing group (EWG) substituents make it sufficiently electron-deficient and electron donating groups (EDGs) raise the dienophile’s filled π orbital’s energy sufficiently to make it the HOMO. Such a scenario is termed an inverse electron demand Diels–Alder reaction. Regardless of which situation pertains, the HOMO and LUMO of the components are in phase and a bonding interaction results as can be seen in the diagram below. Since the reactants are in their ground state, the reaction is initiated thermally and does not require activation by light.
...
Wikipedia
