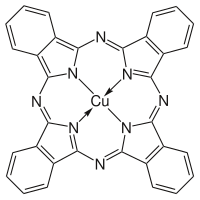
Cu-phthalocyanine
 |
|
| Names | |
|---|---|
| Other names
Monastral blue, phthalo blue, thalo blue
|
|
| Identifiers | |
| Properties | |
| C32H16N8 | |
| Molar mass | 512.54 g·mol−1 |
| Appearance | dark blue solid |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
| Phthalo Blue | |
|---|---|

Phthalocyanine blue pigment powder
|
|
|
|
|
| Hex triplet | #000f89 |
| sRGBB (r, g, b) | (0, 15, 137) |
| CMYKH (c, m, y, k) | (100, 98, 16, 14) |
| HSV (h, s, v) | (233°, 100%, 54%) |
| Source | The Mother of All HTML Colo(u)r Charts |
|
B: Normalized to [0–255] (byte) H: Normalized to [0–100] (hundred) |
|
Phthalocyanine Blue BN, also called by many names (EINECS 205-685-1), is a bright, crystalline, synthetic blue pigment from the group of phthalocyanine dyes. Its brilliant blue is frequently used in paints and dyes. It is highly valued for its superior properties such as light fastness, tinting strength, covering power and resistance to the effects of alkalies and acids. It has the appearance of a blue powder, insoluble in water and most solvents.
CuPc was first prepared in 1927 by the reaction of copper(I) cyanide and o-dibromobenzene, with the apparent intent of preparing phthalonitrile. A couple of years later, workers at Scottish obtained CuPc, FePc, and NiPc from reactions of phthalic anhydride and ammonia. Specifically at the ICI phthalimide plant, a blue contaminant was traced to a by-product formed when the phthalimide reacted with trace amounts of iron from the metal reactor. The chemist took samples of this blue and using sulfuric acid as a solvent, managed to produce a workable pigment. These leads led to the blue pigment sold under the trade name Monastral. Industrial produced commenced in 1935 at ICI, I.G. Farbenindustrie, and DuPont.
Difficulty was experienced in forming stable dispersions with the first alpha forms, especially in mixtures with rutile Titanium, where the blue pigment tended to flocculate. The beta form was more stable, as was the improved stabilized alpha form. Today, there are even more isomeric forms available.
The substance, chemical name (29H,31H-phthalocyaninato(2-)-N29,N30,N31,N32)copper (or copper phthalocyanine), is also known as monastral blue, phthalo blue, helio blue, thalo blue, Winsor blue, phthalocyanine blue, C.I. Pigment Blue 15:2, Copper phthalocyanine blue, Copper tetrabenzoporphyrazine, Cu-Phthaloblue, PB-15, PB-36, C.I. 74160, and British Rail Blue. Numerous other trade names and synonyms exist. The abbreviation "CuPc" is also used.
...
Wikipedia
