Phthalocyanine dyes
 |
|
 |
|
| Names | |
|---|---|
| Other names
Phthalocyanin
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.527 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C32H18N8 | |
| Molar mass | 514.55 g·mol−1 |
| Hazards | |
| S-phrases (outdated) | S22 S24/25 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
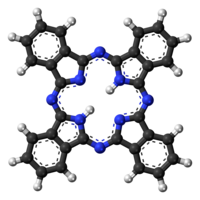
Phthalocyanine is an intensely blue-green-coloured aromatic macrocyclic compound that is widely used in dyeing. Phthalocyanines form coordination complexes with most elements of the periodic table. These complexes are also intensely colored and also are used as dyes or pigments.
Unsubstituted phthalocyanine, abbreviated H
2Pc, and many of its complexes have very low solubility in organic solvents. Benzene at 40 °C dissolves less than a milligram of H
2Pc or CuPc per litre. H
2Pc or CuPc dissolve easily in sulfuric acid due to the protonation of the nitrogen atoms bridging the pyrrole rings. Many phthalocyanine compounds are thermally very stable, do not melt but can be sublimed, CuPc sublimes at >500 °C under inert gases (nitrogen, CO
2). Substituted phthalocyanine complexes often have much higher solubility. They are less thermally stable and often can not be sublimed. Unsubstituted phthalocyanines strongly absorb light between 600 and 700 nm, thus these materials are blue or green. Substitution can shift the absorption towards longer wavelengths, changing the color from pure blue to green to colorless (when the absorption is in the near infrared).
Phthalocyanines are structurally related to other macrocyclic pigments, especially the porphyrins. Both feature four pyrrole-like subunits linked to form a 16-membered ring. The pyrrole-like rings within H
2Pc are closely related to isoindole. Both porphyrins and phthalocyanines function as planar tetradentate dianionic ligands that bind metals through four inwardly projecting nitrogen centers. Such complexes are formally derivatives of Pc2−
, the conjugate base of H
2Pc.
...
Wikipedia
