Cometriq
 |
|
| Clinical data | |
|---|---|
| Trade names | Cabometyx, Cometriq |
| Synonyms | XL184, BMS907351 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | ≥99.7% |
| Metabolism | Hepatic (CYP3A4-mediated) |
| Elimination half-life | 55 hours |
| Excretion | Faeces (54%), urine (27%) |
| Identifiers | |
|
|
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.221.147 |
| Chemical and physical data | |
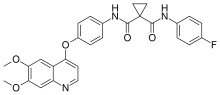
| Formula | C28H24FN3O5 |
| Molar mass | 501.51 g mol |
| 3D model (JSmol) | |
|
|
|
|
Cabozantinib, sold under the brand-name Cabometyx and Cometriq, is a medication used to treat medullary thyroid cancer and a second line treatment for renal cell carcinoma among others. It is a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, and also inhibits AXL and RET. It was discovered and developed by Exelixis Inc.
Cabozantinib is used in two forms. A capsule form is used to treat medullary thyroid cancer and a tablet form is used as a second line treatment for renal cell carcinoma.
Cabozantinib has not been tested in pregnant women; it causes harm to fetuses in rodents. Pregnant women should not take this drug, and women should not become pregnant while taking it. It is not known if cabozantinib is excreted in breast milk.
The drug should be used with caution in people with a history of heart rhythm problems, including long QT interval.
In the US, the capsule formulation (Cometriq) carries a black box warning of the risk of holes forming in the stomach or intestines as well as formation of fistulas (tunnels between the GI tract and the skin). The black box also warns against the risk of uncontrolled bleeding. The tablet formulation (Cabometyx) warns of these effects as well.
The labels also warn of the risk of clots forming and causing heart attacks or strokes, high blood pressure including hypertensive crisis, osteonecrosis of the jaw, severe diarrhea, skin sloughing off the palms and soles, a syndrome with headaches, confusion, loss of vision, and seizures, and protein appearing in urine.
...
Wikipedia
