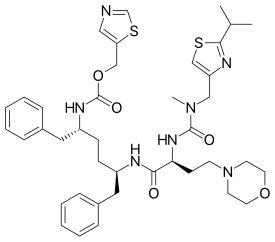
Cobicistat
 |
|
| Clinical data | |
|---|---|
| Trade names | Tybost |
| ATC code | |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C40H53N7O5S2 |
| Molar mass | 776.023 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Cobicistat, with trade name Tybost (formerly GS-9350) is a licensed drug for use in the treatment of human immunodeficiency virus infection (HIV/AIDS).
Like ritonavir (Norvir), cobicistat is of interest for its ability to inhibit liver enzymes that metabolize other medications used to treat HIV, notably elvitegravir, an HIV integrase inhibitor. By combining cobicistat with elvitegravir, higher concentrations of the latter are achieved in the body with lower dosing, theoretically enhancing elvitegravir's viral suppression while diminishing its adverse side-effects. In contrast with ritonavir, the only other booster approved for use as a part of HAART, cobicistat has no anti-HIV activity of its own.
Cobicistat is a component of two four-drug, fixed-dose combination HIV treatments. The first, elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil, is marketed as Stribild and was approved by the FDA in August 2012 for use in the United States. The second, elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide, is marketed as Genvoya and was approved by the FDA in November 2015 for use in the United States. Both Stribild and Genvoya are owned by Gilead Sciences.
Additionally, in existence are a fixed-dose combination of cobicistat and protease inhibitor darunavir (darunavir/cobicistat; marketed as Prezcobix by Janssen Therapeutics), and a fixed-dose combination of cobicistat and protease inhibitor atazanavir (atazanavir/cobicistat; marketed as Evotaz by Bristol-Myers Squibb). Both Prezcobix and Evotaz were approved by the FDA in January 2015.
Cobicistat is a potent inhibitor of 3A enzymes, including the important CYP3A4 subtype. It also inhibits intestinal transport proteins, increasing the overall absorption of several HIV medications, including atazanavir, darunavir, and tenofovir alafenamide.
...
Wikipedia
