Atazanavir
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /ˌæ.tə.ˈzæ.nə.vɪər/, A-tə-ZA-nə-vir |
| Trade names | Reyataz, Evotaz, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603019 |
| Pregnancy category |
|
| Routes of administration |
By mouth |
| ATC code | J05AE08 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60-68% |
| Protein binding | 86% |
| Metabolism | Liver (CYP3A4-mediated) |
| Biological half-life | 6.5 hours |
| Excretion | Fecal and kidney |
| Identifiers | |
|
|
| CAS Number |
198904-31-3 |
| PubChem (CID) | 148192 |
| DrugBank |
DB01072 |
| ChemSpider |
130642 |
| UNII |
QZU4H47A3S |
| KEGG |
D01276 |
| ChEBI |
CHEBI:37924 |
| ChEMBL |
CHEMBL1163 |
| NIAID ChemDB | 057755 |
| Chemical and physical data | |
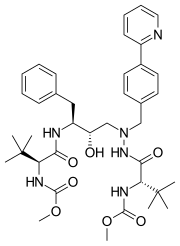
| Formula | C38H52N6O7 |
| Molar mass | 704.856 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
|
|
|
Atazanavir, sold under the trade name Reyataz among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. It is taken by mouth once a day.
Common side effects include headache, nausea, yellowish skin, abdominal pain, trouble sleeping, and fever. Severe side effects include rashes such as erythema multiforme and high blood sugar. Atazanavir appears to be safe to use during pregnancy. It is of the protease inhibitor (PI) class and works by blocking HIV protease.
Atazanavir was approved for medical use in the United States in 2003. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. In the United States it is not available as a generic medication. The wholesale cost in the developing world is about 15.72 USD per month. As of 2015, the cost for a typical month of medication in the United States was more than 200 USD.
Atazanavir is used in the treatment of HIV. The efficacy of atazanavir has been assessed in a number of well designed trials in ART-naive and ART-experienced adults.
Atazanavir is distinguished from other PIs in that it has lesser effects on lipid profile and appears to be less likely to cause lipodystrophy. There may be some cross-resistant with other PIs. When boosted with ritonavir it is equivalent in potency to lopinavir for use in salvage therapy in people with a degree of drug resistance, although boosting with ritonavir reduces the metabolic advantages of atazanavir.
...
Wikipedia
