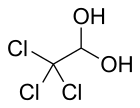
Chloral hydrate
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
2,2,2-Trichloroethane-1,1-diol
|
|
| Other names
Trichloroacetaldehyde monohydrate
Tradenames: Aquachloral, Novo-Chlorhydrate, Somnos, Noctec, Somnote |
|
| Identifiers | |
|
302-17-0 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:28142 |
| ChEMBL |
ChEMBL455917 |
| ChemSpider |
2606 |
| DrugBank |
DB01563 |
| ECHA InfoCard | 100.005.562 |
| EC Number | 206-117-5 |
| KEGG |
D00265 |
| PubChem | 2707 |
| RTECS number | FM875000 |
| UNII |
418M5916WG |
|
|
|
|
| Properties | |
| C2H3Cl3O2 | |
| Molar mass | 165.39 g·mol−1 |
| Appearance | Colorless solid |
| Odor | Aromatic, slightly acrid |
| Density | 1.9081 g/cm3 |
| Melting point | 57 °C (135 °F; 330 K) |
| Boiling point | 98 °C (208 °F; 371 K) |
| very soluble | |
| Solubility | very soluble in benzene, ethyl ether, ethanol |
| log P | 0.99 |
| Acidity (pKa) | 9.66, 11.0 |
| Structure | |
| monoclinic | |
| Pharmacology | |
| N05CC01 (WHO) | |
|
|
| Oral syrup, rectal suppository | |
| Pharmacokinetics: | |
| Well absorbed | |
| Hepatic and renal (Converted to trichloroethanol) | |
| 8–10 hours | |
| Bile, feces, urine (various metabolites not unchanged) | |
| Legal status |
|
| Hazards | |
| Safety data sheet | External MSDS |
|
EU classification (DSD)
|
Harmful (Xn) |
| R-phrases | R22 R36 R37 R38 |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
1100 mg/kg (mouse, oral) |
| Related compounds | |
|
Related compounds
|
Chloral, chlorobutanol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Chloral hydrate is an organic compound with the formula C2H3Cl3O2. It is a colorless solid. It has limited use as a sedative and hypnotic pharmaceutical drug. It is also a useful laboratory chemical reagent and precursor. It is derived from chloral (trichloroacetaldehyde) by the addition of one equivalent of water.
It was discovered through the chlorination (halogenation) of ethanol in 1832 by Justus von Liebig in Gießen. Its sedative properties were first published in 1869 and subsequently, because of its easy synthesis, its use was widespread. It was widely used recreationally and misprescribed in the late 19th century. Chloral hydrate is soluble in both water and ethanol, readily forming concentrated solutions. A solution of chloral hydrate in ethanol called "knockout drops" was used to prepare a Mickey Finn. More reputable uses of chloral hydrate include its use as a clearing agent for chitin and fibers and as a key ingredient in Hoyer's mounting medium, which is used to prepare permanent or semi-permanent microscope slides of small organisms, histological sections, and chromosome squashes. Because of its status as a regulated substance, chloral hydrate can be difficult to obtain. This has led to chloral hydrate being replaced by alternative reagents in microscopy procedures.
It is, together with chloroform, a minor side-product of the chlorination of water when organic residues such as humic acids are present. It has been detected in drinking water at concentrations of up to 100 micrograms per litre (µg/L) but concentrations are normally found to be below 10 µg/L. Levels are generally found to be higher in surface water than in ground water.
...
Wikipedia
