Chichibabin pyridine synthesis
| Chichibabin pyridine synthesis | |
|---|---|
| Named after | Aleksei Chichibabin |
| Reaction type | Ring forming reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000526 |
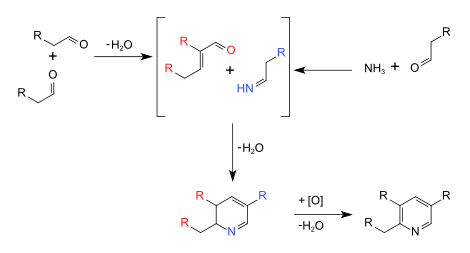
The Chichibabin pyridine synthesis (/ˈtʃitʃiˌbeɪbin/ CHEE-chee-BAY-been) is a method for synthesizing pyridine rings. In its general form, the reaction can be described as a condensation reaction of aldehydes, ketones, α,β-Unsaturated carbonyl compounds, or any combination of the above, in ammonia or ammonia derivatives. It was reported by Aleksei Chichibabin in 1924. The following is the overall form of the general reaction:
The elementary contributing steps of the reaction mechanism can be classified as more familiar name reactions, including an imine synthesis, a base-catalyzed aldol condensation, and initiating the ring-synthesis step, a Michael reaction.
...
Wikipedia