Carvedilol
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
|
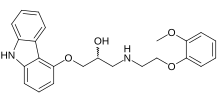
(±)-[3-(9H-carbazol-4-yloxy)-2-hydroxypropyl][2-(2-methoxyphenoxy)ethyl]amine
|
|
| Clinical data | |
| Trade names | Coreg |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697042 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 25–35% |
| Protein binding | 98% |
| Metabolism | Liver (CYP2D6, CYP2C9) |
| Biological half-life | 7–10 hours |
| Excretion | Urine (16%), Feces (60%) |
| Identifiers | |
| CAS Number |
72956-09-3 |
| ATC code | C07AG02 (WHO) |
| PubChem | CID 2585 |
| IUPHAR/BPS | 551 |
| DrugBank |
DB01136 |
| ChemSpider |
2487 |
| UNII |
0K47UL67F2 |
| KEGG |
D00255 |
| ChEBI |
CHEBI:3441 |
| ChEMBL |
CHEMBL723 |
| PDB ligand ID | CVD (PDBe, RCSB PDB) |
| Chemical data | |
| Formula | C24H26N2O4 |
| Molar mass | 406.474 |
| Chirality | Racemic mixture |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Carvedilol, sold under the brand name Coreg among others, is a beta blocker used for treating mild to severe congestive heart failure (CHF), left ventricular dysfunction (LVD) following heart attack in people who are otherwise stable, and for treating high blood pressure.
Beta blockers block the beta receptors on heart muscle and other cells, making them more relaxed and less responsive to stress hormones. Carvedilol also blocks alpha receptors, which are found on blood vessels, and relaxes the blood vessels, dilating them, which lowers blood pressure and vascular resistance.
It is a nonselective beta blocker/alpha-1 blocker and belongs to the third generation of betablockers.
Carvedilol was discovered by Fritz Wiedemann at Boehringer Mannheim and was initially approved in the U.S. in 1995. On October 20, 2006, the FDA approved an extended-release formulation.
Carvedilol is indicated in the management of congestive heart failure (CHF), commonly as an adjunct to angiotensin-converting-enzyme inhibitor (ACE inhibitors) and diuretics. It has been clinically shown to reduce mortality and hospitalizations in people with CHF. The mechanism behind its positive effect when used long-term in clinically stable CHF patients is not fully understood, but is thought to contribute to remodeling of the heart, improving upon its structure and function.
In addition, carvedilol is indicated in the treatment of hypertension and to reduce risk of mortality and hospitalizations in a subset of people following a heart attack. It can be used alone or with other anti-hypertensive agents. In the 2013 guideline, it is recommended as the drug of choice in people with histories of CHF and/or myocardial infarction.
The most common side effects (>10% incidence) include:
Carvedilol is not recommended for people with uncontrolled bronchospastic disease (e.g. current asthma symptoms) as it can block receptors that assist in opening the airways.
Carvedilol may mask symptoms of low blood sugar.
According to the FDA, carvedilol should not be used in people with bronchial asthma or bronchospastic conditions. It should not be used in people with second- or third-degree AV block, sick sinus syndrome, severe bradycardia (unless a permanent pacemaker is in place), or a decompensated heart condition. People with severe hepatic impairment are also not advised to take carvedilol.
...
Wikipedia
