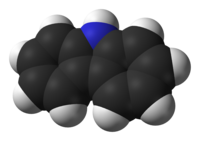
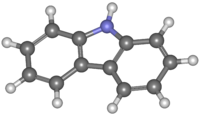
Carbazole
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
9H-carbazole
|
|
| Other names
9-azafluorene, dibenzopyrrole, diphenylenimine, diphenyleneimide, USAF EK-600
|
|
| Identifiers | |
|
86-74-8 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:27543 |
| ChEMBL |
ChEMBL243580 |
| ChemSpider |
6593 |
| DrugBank |
DB07301 |
| ECHA InfoCard | 100.001.542 |
| KEGG |
C08060 |
| PubChem | 6854 |
| UNII |
0P2197HHHN |
|
|
|
|
| Properties | |
| C12H9N | |
| Molar mass | 167.206 g mol−1 |
| Density | 1.301g/cm^3 |
| Melting point | 246.3 °C (475.3 °F; 519.5 K) |
| Boiling point | 354.69 °C (670.44 °F; 627.84 K) |
| -117.4·10−6 cm3/mol | |
| Hazards | |
| Flash point | 220 °C (428 °F; 493 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. The compound's structure is based on the indole structure, but in which a second benzene ring is fused onto the five-membered ring at the 2–3 position of indole (equivalent to the 9a–4a double bond in carbazole, respectively).
A classic laboratory organic synthesis for carbazole is the Borsche–Drechsel cyclization.
In the first step, phenylhydrazine is condensed with cyclohexanone to the corresponding imine. The second step is a hydrochloric acid-catalyzed rearrangement reaction and ring-closing reaction to tetrahydrocarbazole. In one modification, both steps are rolled into one by carrying out the reaction in acetic acid. In the third step, this compound is oxidized by red lead to carbazole itself. Another classic is the Bucherer carbazole synthesis
A second method for the synthesis of carbazole is the Graebe–Ullmann reaction.
In the first step, an N-phenyl-1,2-diaminobenzene (N-phenyl-o-phenylenediamine) is converted into a diazonium salt which instantaneously forms a 1,2,3-triazole. The triazole is unstable and at elevated temperatures, nitrogen is set free and the carbazole is formed.
...
Wikipedia
