Blood lactate
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
2-Hydroxypropanoic acid
|
|||
| Other names
Lactic acid
Milk acid |
|||
| Identifiers | |||
|
|||
|
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.017 | ||
| E number | E270 (preservatives) | ||
| UNII | |||
|
|||
|
|||
| Properties | |||
| C3H6O3 | |||
| Molar mass | 90.08 g·mol−1 | ||
| Melting point | 53°C | ||
| Boiling point | 122 °C (252 °F; 395 K) @ 15 mmHg | ||
| Acidity (pKa) | 3.86, 15.1 | ||
| Thermochemistry | |||
|
Std enthalpy of
combustion (ΔcH |
1361.9 kJ/mol, 325.5 kcal/mol, 15.1 kJ/g, 3.61 kcal/g | ||
| Pharmacology | |||
| G01AD01 (WHO) QP53AG02 (WHO) | |||
| Hazards | |||
| GHS pictograms |  |
||
| H315, H318 | |||
| P280, P305+351+338 | |||
| Related compounds | |||
|
Other anions
|
lactate | ||
|
Related carboxylic acids
|
acetic acid glycolic acid propionic acid 3-hydroxypropanoic acid malonic acid butyric acid hydroxybutyric acid |
||
|
Related compounds
|
1-propanol 2-propanol propionaldehyde acrolein sodium lactate |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
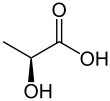
Lactic acid is an organic compound with the formula CH3CH(OH)COOH. In its solid state, it is white and water-soluble. In its liquid state, it is colorless. It is produced both naturally and synthetically. With a hydroxyl group adjacent to the carboxyl group, lactic acid is classified as an alpha-hydroxy acid (AHA). In the form of its conjugate base called lactate, it plays a role in several biochemical processes.
In solution, it can ionize a proton from the carboxyl group, producing the lactate ion CH
3CH(OH)CO−
2. Compared to acetic acid, its pKa is 1 unit less, meaning lactic acid deprotonates ten times more easily than acetic acid does. This higher acidity is the consequence of the intramolecular hydrogen bonding between the α-hydroxyl and the carboxylate group.
Lactic acid is chiral, consisting of two optical isomers. One is known as L-(+)-lactic acid or (S)-lactic acid and the other, its mirror image, is D-(−)-lactic acid or (R)-lactic acid. A mixture of the two in equal amounts is called DL-lactic acid, or racemic lactic acid.
Lactic acid is hygroscopic. DL-lactic acid is miscible with water and with ethanol above its melting point which is around 17 or 18 °C. D-lactic acid and L-lactic acid have a higher melting point.
In animals, L-lactate is constantly produced from pyruvate via the enzyme lactate dehydrogenase (LDH) in a process of fermentation during normal metabolism and exercise. It does not increase in concentration until the rate of lactate production exceeds the rate of lactate removal, which is governed by a number of factors, including monocarboxylate transporters, concentration and isoform of LDH, and oxidative capacity of tissues. The concentration of blood lactate is usually 1–2 mmol/L at rest, but can rise to over 20 mmol/L during intense exertion and as high as 25 mmol/L afterward.
...
Wikipedia