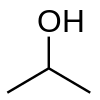
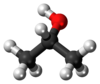
2-propanol
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Propan-2-ol
|
|||
| Other names
2-Propanol
Isopropanol (incorrect) Rubbing alcohol sec-Propyl alcohol s-Propanol iPrOH Dimethyl carbinol IPA |
|||
| Identifiers | |||
|
67-63-0 |
|||
| 3D model (Jmol) | Interactive image | ||
| 635639 | |||
| ChEBI |
CHEBI:17824 |
||
| ChEMBL |
ChEMBL582 |
||
| ChemSpider |
3644 |
||
| ECHA InfoCard | 100.000.601 | ||
| 1464 | |||
| KEGG |
D00137 |
||
| PubChem | 3776 | ||
| RTECS number | NT8050000 | ||
| UNII |
ND2M416302 |
||
| UN number | 1219 | ||
|
|||
|
|||
| Properties | |||
| C3H8O | |||
| Molar mass | 60.10 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.786 g/cm3 (20 °C) | ||
| Melting point | −89 °C (−128 °F; 184 K) | ||
| Boiling point | 82.6 °C (180.7 °F; 355.8 K) | ||
| miscible in water | |||
| Solubility | miscible in benzene, chloroform, ethanol, ether, glycerin soluble in acetone |
||
| Acidity (pKa) | 16.5 | ||
| -45.794·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.3776 | ||
| Viscosity | 2.86 cP at 15 °C 1.96 cP at 25 °C 1.77 cP at 30 °C |
||
| 1.66 D (gas) | |||
| Pharmacology | |||
| D08AX05 (WHO) | |||
| Hazards | |||
| Main hazards | Flammable | ||
| Safety data sheet |
See: data page External MSDS |
||
| GHS pictograms |
 
|
||
| GHS signal word | Danger | ||
| H225, H319, H336 | |||
| P210, P261, P305+351+338 | |||
|
EU classification (DSD)
|
Highly flammable (F), Irritating (Xi) | ||
| R-phrases | R11 R36 R67 | ||
| S-phrases | S7 S16 S24 S25 S26 | ||
| NFPA 704 | |||
| Flash point | Open cup: 11.7 °C (53.1 °F; 284.8 K) Closed cup: 13 °C (55 °F) |
||
| 399 °C (750 °F; 672 K) | |||
| Explosive limits | 2–12.7% | ||
| 980 mg/m3 (TWA), 1225 mg/m3 (STEL) | |||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
12800 mg/kg (dermal, rabbit) 3600 mg/kg (oral, mouse) 5045 mg/kg (oral, rat) 6410 mg/kg (oral, rabbit) |
||
|
LC50 (median concentration)
|
53000 mg/m3 (inhalation, mouse) 12,000 ppm (rat, 8 hr) |
||
|
LCLo (lowest published)
|
16,000 ppm (rat, 4 hr) 12,800 ppm (mouse, 3 hr) |
||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 400 ppm (980 mg/m3) | ||
|
REL (Recommended)
|
TWA 400 ppm (980 mg/m3) ST 500 ppm (1225 mg/m3) | ||
|
IDLH (Immediate danger)
|
2000 ppm | ||
| Related compounds | |||
|
Related alcohols
|
1-Propanol, ethanol, 2-butanol | ||
| Supplementary data page | |||
|
Refractive index (n), Dielectric constant (εr), etc. |
|||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
| UV, IR, NMR, MS | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Isopropyl alcohol (IUPAC name propan-2-ol), also called isopropanol or dimethyl carbinol, is a compound with the chemical formula C3H8O or C3H7OH or CH3CHOHCH3 (sometimes represented as i-PrOH). It is a colorless, flammable chemical compound with a strong odor. As a propyl group linked to a hydroxyl group, it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms, sometimes shown as (CH3)2CHOH. It is a structural isomer of 1-propanol. It has a wide variety of industrial and household uses.
Isopropyl alcohol is miscible in water, ethanol, ether, and chloroform. It will dissolve ethyl cellulose, polyvinyl butyral, many oils, alkaloids, gums and natural resins. Unlike ethanol or methanol, isopropyl alcohol is not miscible with salt solutions and can be separated from aqueous solutions by adding a salt such as sodium chloride. The process is colloquially called salting out, and causes concentrated isopropyl alcohol to separate into a distinct layer.
Isopropyl alcohol forms an azeotrope with water, which gives a boiling point of 80.37 °C (176.67 °F) and a composition of 87.7 wt% (91 vol%) isopropyl alcohol. Water-isopropyl alcohol mixtures have depressed melting points. It has a slightly bitter taste, and is not safe to drink.
...
Wikipedia