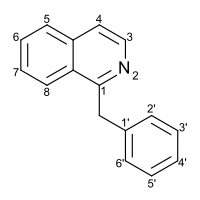
Benzylisoquinoline
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
1-Benzylisoquinoline
|
|
|
Systematic IUPAC name
1-(Phenylmethyl)isoquinoline
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C16H13N | |
| Molar mass | 219.28112 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
1-Benzylisoquinoline is a chemical compound, and the structural backbone of many alkaloids with a wide variety of structures, including papaverine, noscapine, codeine, morphine, apomorphine, berberine, protopine, tubocurarine, and sanguinarine.
Plants producing benzylisoquinoline alkaloids have a common biosynthetic pathway, making use of two units of L-tyrosine. One tyrosine molecule is metabolised to dopamine which constitutes the isoquinoline part, while the benzylic part is mostly formed from tyramine, itself the decarboxylation product of tyrosine.
Many benzylisoquinolines have a methylated nitrogen atom as well as functional groups containing oxygen (−OH, −OCH3, −OCH2O−) in positions 6, 7, 3′ and 4′. The latter come from the precursors mentioned above, namely tyrosine, dopamine and their derivatives.
Apomorphine (one additional ring closure)
Morphine (two additional ring closures)
Berberine (one additional ring closure with incorporated N-methyl)
Protopine (with opened pyridine ring)
Tubocurarine (composed of two benzylisoquinoline units)
...
Wikipedia
