Tyrosine

L-Tyrosine
|
|
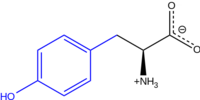
L-Tyrosine at physiological pH
|
|
| Names | |
|---|---|
|
IUPAC name
(S)-Tyrosine
|
|
| Other names
L-2-Amino-3-(4-hydroxyphenyl)propanoic acid
|
|
| Identifiers | |
|
60-18-4 (L) |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:58315 |
| ChEMBL |
ChEMBL925 |
| ChemSpider |
5833 |
| DrugBank |
DB03839 |
| ECHA InfoCard | 100.000.419 |
| 4791 | |
| PubChem | 1153 |
| UNII |
42HK56048U |
|
|
|
|
| Properties | |
| C9H11NO3 | |
| Molar mass | 181.19 g·mol−1 |
| -105.3·10−6 cm3/mol | |
| Hazards | |
| Safety data sheet | See: data page |
| NFPA 704 | |
| Supplementary data page | |
|
Refractive index (n), Dielectric constant (εr), etc. |
|
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Tyrosine (Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. Its codons are UAC and UAU. The word "tyrosine" is from the Greek tyros, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. Tyrosine is a hydrophilic amino acid and is significantly more soluble in water than its precursor, phenylalanine, due to the thermodynamic favorability of the hydrogen bonding between the hydroxyl group of one molecule of tyrosine and the carboxyl group of another.
Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes. It functions as a receiver of phosphate groups that are transferred by way of protein kinases (so-called receptor tyrosine kinases). Phosphorylation of the hydroxyl group changes the activity of the target protein.
A tyrosine residue also plays an important role in photosynthesis. In chloroplasts (photosystem II), it acts as an electron donor in the reduction of oxidized chlorophyll. In this process, it loses the hydrogen atom of its phenolic OH-group. This radical is subsequently reduced in the photosystem II by the four core manganese clusters.
...
Wikipedia

