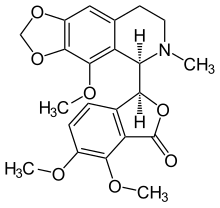
Noscapine
 |
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~30% |
| Biological half-life | 1.5 to 4h (mean 2.5) |
| Identifiers | |
|
|
| Synonyms | Narcotine |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.004.455 |
| Chemical and physical data | |
| Formula | C22H23NO7 |
| Molar mass | 413.421 |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Noscapine (also known as Narcotine, Nectodon, Nospen, Anarcotine and (archaic) Opiane) is a benzylisoquinoline alkaloid from plants of the poppy family, without painkilling properties. This agent is primarily used for its antitussive (cough-suppressing) effects.
Noscapine is often used as an antitussive medication. A 2012 Dutch guideline, however, does not recommend its use for coughing. Noscapine also has anti-cancer properties, inhibiting microtubule interactions, thereby stopping cell proliferation.
Noscapine can increase the effects of centrally sedating substances such as alcohol and hypnotics.
The drug should not be taken with any MAOIs (monoamine oxidase inhibitors), as unknown and potentially fatal effects may occur.
Noscapine should not be taken in conjunction with warfarin as the anticoagulant effects of warfarin may be increased.
Noscapine's antitussive effects appear to be primarily mediated by its σ–receptor agonist activity. Evidence for this mechanism is suggested by experimental evidence in rats. Pretreatment with rimcazole, a σ-specific antagonist, causes a dose-dependent reduction in antitussive activity of noscapine. Noscapine, and its synthetic derivatives called noscapinoids, are known to interact with microtubules and inhibit cancer cell proliferation
The lactone ring is unstable and opens in basic media. The opposite reaction is presented in acidic media. The bond (C1−C3′) connecting the two optically active carbon atoms is also unstable. In aqueous solution of sulfuric acid and heating it dissociates into cotarnine (4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinoline) and opic acid (6-formyl-2,3-dimethoxybenzoic acid). When noscapine is reduced with zinc/HCl, the bond C1−C3′ saturates and the molecule dissociates into hydrocotarnine (2-hydroxycotarnine) and meconine (6,7-dimethoxyisobenzofuran-1(3H)-one).
...
Wikipedia
