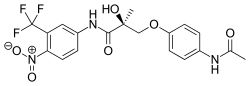
Andarine
 |
|
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
|
|
| Synonyms | Acetamidoxolutamide; Androxolutamide; GTx-007; S-4 |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| ChEMBL | |
| ECHA InfoCard | 100.230.653 |
| Chemical and physical data | |
| Formula | C19H18F3N3O6 |
| Molar mass | 441.357 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Andarine (GTx-007, S-4) is an investigational selective androgen receptor modulator (SARM) developed by GTX, Inc for treatment of conditions such as muscle wasting, osteoporosis and benign prostatic hypertrophy, using the non-steroidal androgen antagonist bicalutamide as a lead compound.
Andarine is an orally active partial agonist for androgen receptors. It is less potent in both anabolic and androgenic effects than other SARMs. In an animal model of benign prostatic hypertrophy, andarine was shown to reduce prostate weight with similar efficacy to finasteride, but without producing any reduction in muscle mass or anti-androgenic side effects. This suggests that it is able to competitively block binding of dihydrotestosterone to its receptor targets in the prostate gland, but its partial agonist effects at androgen receptors prevent the side effects associated with the anti-androgenic drugs traditionally used for treatment of BPH.
...
Wikipedia
