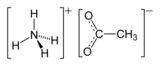
Ammonium acetate
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Ammonium ethanoate
|
|
| Identifiers | |
|
631-61-8 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:62947 |
| ChemSpider |
11925 |
| ECHA InfoCard | 100.010.149 |
| PubChem | 517165 |
| RTECS number | AF3675000 |
| UNII |
RRE756S6Q2 |
|
|
|
|
| Properties | |
| C2H7NO2 | |
| Molar mass | 77.08 g·mol−1 |
| Appearance | White solid crystals, deliquescent |
| Odor | Slightly acetic |
| Density | 1.17 g/cm3 (20 °C) 1.073 g/cm3 (25 °C) |
| Melting point | 113 °C (235 °F; 386 K) |
| 102 g/100 mL (0 °C) 148 g/100 mL (4 °C) 143 g/100 mL (20 °C) 533 g/100 mL (80 °C) |
|
| Solubility | Soluble in alcohol, SO2, acetone, liquid ammonia |
| Solubility in methanol | 7.89 g/100 mL (15 °C) 131.24 g/100 g (94.2 °C) |
| Solubility in dimethylformamide | 0.1 g/100 g |
| -41.1·10−6 cm3/mol | |
| Structure | |
| Orthorhombic | |
| Thermochemistry | |
|
Std enthalpy of
formation (ΔfH |
−615 kJ/mol |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | JT Baker |
| GHS pictograms |  |
| GHS signal word | Warning |
| H303, H316, H320, H333 | |
| P281, P335 | |
| NFPA 704 | |
| Flash point | 136 °C (277 °F; 409 K) |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
386 mg/kg (mice, intravenous) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Ammonium acetate is a chemical compound with the formula NH4CH3CO2. It is a white, hygroscopic solid and can be derived from the reaction of ammonia and acetic acid. It is available commercially.
It is the main precursor to acetamide:
It is also used as a diuretic.
As the salt of a weak acid and a weak base, ammonium acetate is often used with acetic acid to create a buffer solution. Ammonium acetate is volatile at low pressures. Because of this, it has been used to replace cell buffers with non-volatile salts in preparing samples for mass spectrometry. It is also popular as a buffer for mobile phases for HPLC with ELSD detection for this reason. Other volatile salts that have been used for this include ammonium formate.
Ammonium acetate is also used as a food additive as an acidity regulator; INS number 264. It is approved for usage in Australia and New Zealand.
Ammonium acetate is produced by the neutralization of acetic acid with ammonium carbonate or by saturating glacial acetic acid with ammonia. Obtaining crystalline ammonium acetate is difficult on account of its aqueous solution giving off ammonia when evaporated.
...
Wikipedia

