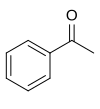
Acetophenone
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
1-Phenylethan-1-one
|
|||
| Other names
Acetophenone
Methyl phenyl ketone Phenylethanone |
|||
| Identifiers | |||
|
98-86-2 |
|||
| 3D model (Jmol) |
Interactive image Interactive image |
||
| Abbreviations | ACP | ||
| ChEBI |
CHEBI:27632 |
||
| ChEMBL |
ChEMBL274467 |
||
| ChemSpider |
7132 |
||
| DrugBank |
DB04619 |
||
| ECHA InfoCard | 100.002.462 | ||
| KEGG |
C07113 |
||
| PubChem | 7410 | ||
| UNII |
RK493WHV10 |
||
|
|||
|
|||
| Properties | |||
| C8H8O | |||
| Molar mass | 120.15 g·mol−1 | ||
| Density | 1.028 g/cm3 | ||
| Melting point | 19–20 °C (66–68 °F; 292–293 K) | ||
| Boiling point | 202 °C (396 °F; 475 K) | ||
| 5.5 g/L at 25 °C 12.2 g/L at 80 °C |
|||
| -72.05·10−6 cm3/mol | |||
| Hazards | |||
| Safety data sheet | MSDS | ||
|
EU classification (DSD)
|
|||
| NFPA 704 | |||
| Flash point | 77 °C (171 °F; 350 K) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Acetophenone is the organic compound with the formula C6H5C(O)CH3 (also represented by the letters PhAc or BzMe), is the simplest aromatic ketone. This colourless, viscous liquid is a precursor to useful resins and fragrances.
Acetophenone can be obtained by a variety of methods. In industry, acetophenone is recovered as a by-product of the oxidation of ethylbenzene, which mainly gives ethylbenzene hydroperoxide for use in the production of propylene oxide.
Commercially significant resins are produced from treatment of acetophenone with formaldehyde and a base. The resulting copolymers are conventionally described with the formula [(C6H5COCH)x(CH2)x]n, resulting from aldol condensation. These substances are components of coatings and inks. Modified acetophenone-formaldehyde resins are produced by the hydrogenation of the aforementioned ketone-containing resins. The resulting polyol can be further crosslinked with diisocyanates. These modified resins are again found in coatings, inks, as well as adhesives.
Acetophenone is an ingredient in fragrances that resemble almond, cherry, honeysuckle, jasmine, and strawberry. It is used in chewing gum. It is also listed as an approved excipient by the U.S. FDA. In a 1994 report released by five top cigarette companies in the U.S., acetophenone was listed as one of the 599 additives to cigarettes.
...
Wikipedia