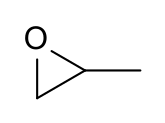
Propylene oxide
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
2-Methyloxirane
|
|
| Other names
Propylene oxide
Epoxypropane Propylene epoxide 1,2-Propylene oxide Methyl oxirane 1,2-Epoxypropane Propene oxide Methyl ethylene oxide Methylethylene oxide |
|
| Identifiers | |
|
75-56-9 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:38685 |
| ECHA InfoCard | 100.000.800 |
| EC Number | 200-879-2 |
| KEGG |
C15508 |
|
|
| Properties | |
| C3H6O | |
| Molar mass | 58.08 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | benzene-like |
| Density | 0.830 g/cm3 |
| Melting point | −112 °C (−170 °F; 161 K) |
| Boiling point | 34 °C (93 °F; 307 K) |
| 41% (20°C) | |
| Vapor pressure | 445 mmHg (20°C) |
| -42.5·10−6 cm3/mol | |
| Hazards | |
| Main hazards | Extremely flammable |
| Safety data sheet | Oxford MSDS |
| GHS pictograms |
  
|
| GHS signal word | DANGER! |
| R-phrases | R45, R46, R12, R20/21/22, R36/37/38 |
| S-phrases | S53, S45 |
| NFPA 704 | |
| Flash point | −37 °C (−35 °F; 236 K) |
| 747 °C (1,377 °F; 1,020 K) | |
| Explosive limits | 2.3-36% |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
660 mg/kg (guinea pig, oral) 380 mg/kg (rat, oral) 440 mg/kg (mouse, oral) 1140 mg/kg (rat, oral) 690 mg/kg (guinea pig, oral) |
|
LC50 (median concentration)
|
1740 ppm (mouse, 4 hr) 4000 ppm (rat, 4 hr) |
|
LCLo (lowest published)
|
2005 ppm (dog, 4 hr) 4000 ppm (guinea pig, 4 hr) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 100 ppm (240 mg/m3) |
|
REL (Recommended)
|
Ca |
|
IDLH (Immediate danger)
|
Ca [400 ppm] |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Propylene oxide is an organic compound with the molecular formula CH3CHCH2O. This colourless volatile liquid is produced on a large scale industrially, its major application being its use for the production of polyether polyols for use in making polyurethane plastics. It is a chiral epoxide, although it is commonly used as a racemic mixture.
This compound is sometimes called 1,2-propylene oxide to distinguish it from its isomer 1,3-propylene oxide, better known as oxetane.
Industrial production of propylene oxide starts from propylene. Two general approaches are employed, one involving hydrochlorination and the other involving oxidation. In 2005, about half of the world production was through chlorohydrin technology and one half via oxidation routes. The latter approach is growing in importance.
The traditional route proceeds via the conversion of propylene to propylene chlorohydrin, which is produced according to the following simplified scheme:
The mixture of 1-chloro-2-propanol and 2-chloro-1-propanol, which is then dehydrochlorinated. For example:
Lime (Ca(OH)2) is often used to absorb the HCl.
The other general route to propylene oxide involves oxidation of propylene with an organic peroxide. The reaction follows this stoichiometry:
The process is practiced with three hydroperoxide]]s:
In March 2009, BASF and Dow Chemical started up their new HPPO plant in Antwerp. In the HPPO-Process, propylene is oxidized with hydrogen peroxide:
In this process no side products other than water are generated.
Between 60 and 70% of all propylene oxide is converted to polyether polyols by the process called alkoxylation. These polyols are building blocks in the production of polyurethane plastics. About 20% of propylene oxide is hydrolyzed into propylene glycol, via a process which is accelerated by acid or base catalysis. Other major products are polypropylene glycol, propylene glycol ethers, and propylene carbonate.
...
Wikipedia

