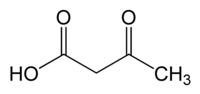
Acetoacetate
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
3-Oxobutanoic acid
|
|
| Other names
Acetoacetic acid (no longer recommended)
Diacetic acid |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C4H6O3 | |
| Molar mass | 102.088 g/mol |
| Appearance | colorless, oily liquid |
| Melting point | 36.5 °C (97.7 °F; 309.6 K) |
| Boiling point | Decomposes |
| soluble | |
| Solubility | soluble in ethanol, ether |
| Acidity (pKa) | 3.58 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Acetoacetic acid (also diacetic acid) is the organic compound with the formula CH3COCH2COOH. It is the simplest beta-keto acid group, and like other members of this class it is unstable. The methyl and ethyl esters, which are quite stable, are produced on a large scale industrially as precursors to dyes.
Acetoacetic acid is a weak acid. It is of biochemical importance in various animals, including humans, as one of the endogenous ketone bodies produced by the liver when it breaks down fatty acids into Acetyl-CoA and TCA cycle intermediates are depleted (particularly oxaloacetate, which is formed from pyruvate derived from glycolysis). It can be viewed as the product of joining two acetic acid molecules via a condensation reaction that ejects a water molecule in the process, although that is only one of the ways of forming the acetoacetate molecule. In the human body, a large portion of acetoacetate is converted to beta-hydroxybutyrate, a rich energy source for the brain, which cannot run directly on fatty acids themselves due to their poor ability to cross the blood-brain barrier.
In general, the esters are prepared from diketene by treatment with alcohols. Acetoacetic acid can be prepared by the hydrolysis of the ethyl acetoacetate followed by acidification of the anion. In general, acetoacetic acid is generated at 0 °C and used in situ immediately. It decomposes at a moderate rate to acetone and carbon dioxide:
...
Wikipedia
