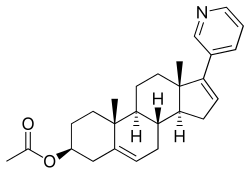
Abiraterone acetate
 |
|
| Clinical data | |
|---|---|
| Trade names | Zytiga |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611046 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral (tablets) |
| ATC code | L02BX03 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | >99% |
| Metabolism | Esterases, CYP3A4, SULT2A1 |
| Biological half-life | 12 ± 5 hours |
| Excretion | Feces (88%), urine (5%) |
| Identifiers | |
|
|
| CAS Number |
154229-19-3 |
| PubChem (CID) | 132971 |
| IUPHAR/BPS | 6745 |
| ChemSpider |
117349 |
| UNII |
G819A456D0 |
| ChEMBL |
CHEMBL254328 |
| Chemical and physical data | |
| Formula | C24H31NO |
| Molar mass | 349.509 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
|
|
|
Abiraterone acetate (INN, USAN, BAN, JAN) (brand names Zytiga, Abiratas, Abretone, Abirapro) is a steroidal CYP17A1 inhibitor and by extension androgen synthesis inhibitor which is used in combination with prednisone in metastatic castration-resistant prostate cancer (previously called hormone-resistant or hormone-refractory prostate cancer) – i.e., prostate cancer not responding to androgen deprivation or treatment with androgen receptor antagonists. It is a prodrug to the active agent abiraterone, and is marketed by Janssen Biotech under the trade name Zytiga. In addition, Intas Pharmaceuticals markets the drug under the trade name Abiratas, Cadila Pharmaceuticals markets the drug as Abretone, and Glenmark Pharmaceuticals as Abirapro.
Abiraterone acetate was approved by the United States Food and Drug Administration on April 28, 2011. The FDA press release made reference to a phase III clinical trial in which abiraterone use was associated with a median survival of 14.8 months versus 10.9 months with placebo; the study was stopped early because of the successful outcome.
...
Wikipedia
