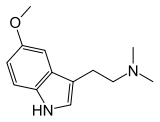
5-methoxy-dimethyltryptamine
 |
|
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Smoked, Insufflated, Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.012.558 |
| Chemical and physical data | |
| Formula | C13H18N2O |
| Molar mass | 218.30 g·mol−1 |
| 3D model (JSmol) | |
|
|
|
|
|
|
|
5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine) is a psychedelic of the tryptamine class. It is found in a wide variety of plant species, and a single psychoactive toad species, the Colorado River toad. Like its close relatives DMT and bufotenin (5-HO-DMT), it has been used as an entheogen in South America.
5-MeO-DMT was first synthesized in 1936, and in 1959 it was isolated as one of the psychoactive ingredients of Anadenanthera peregrina seeds used in preparing Yopo snuff. It was once believed to be a major component of the psychoactive effects of the snuff, although this has recently been shown to be unlikely, due to the limited or sometimes even non-existent quantity contained within the seeds, which instead achieve their psychoactivity from the O-demethylated metabolite of 5-MeO-DMT, bufotenin. It is metabolized mainly by CYP2D6.
5-MeO-DMT is a sacrament of the Church of the Tree of Life. From approximately 1971 to the late 1980s, 5-MeO-DMT was discreetly available to its members. Between 1970 and 1990 smoking of 5-MeO-DMT on parsley was probably one of the two most common forms of ingestion in the United States.
...
Wikipedia
