Silver oxide
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Silver(I) oxide
|
|
| Other names
Silver rust, Argentous oxide, Silver monoxide
|
|
| Identifiers | |
|
20667-12-3 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
7970393 |
| ECHA InfoCard | 100.039.946 |
| EC Number | 243-957-1 |
| MeSH | silver+oxide |
| PubChem | 9794626 |
| RTECS number | VW4900000 |
|
|
|
|
| Properties | |
| Ag2O | |
| Molar mass | 231.74 g·mol−1 |
| Appearance | Black/ brown cubic crystals |
| Odor | Odorless |
| Density | 7.14 g/cm3 |
| Melting point | 300 °C (572 °F; 573 K) decomposes from ≥200 °C |
| 0.013 g/L (20 °C) 0.025 g/L (25 °C) 0.053 g/L (80 °C) |
|
|
Solubility product (Ksp) of AgOH
|
1.52·10−8 (20 °C) |
| Solubility | Soluble in acid, alkali Insoluble in ethanol |
| −134.0·10−6 cm3/mol | |
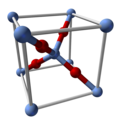
| Structure | |
| Cubic | |
| Pn3m, 224 | |
| Thermochemistry | |
| 65.9 J/mol·K | |
|
Std molar
entropy (S |
122 J/mol·K |
|
Std enthalpy of
formation (ΔfH |
−31 kJ/mol |
|
Gibbs free energy (ΔfG˚)
|
−11.3 kJ/mol |
| Hazards | |
| GHS pictograms |
 
|
| GHS signal word | Danger |
| H272, H315, H319, H335 | |
| P220, P261, P305+351+338 | |
|
EU classification (DSD)
|
|
| R-phrases | R36/37/38 |
| S-phrases | S17, S26, S36 |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
2.82 g/kg (rats, oral) |
| Related compounds | |
|
Related compounds
|
Silver(I,III) oxide |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Silver(I) oxide is the chemical compound with the formula Ag2O. It is a fine black or dark brown powder that is used to prepare other silver compounds.
Silver oxide can be prepared by combining aqueous solutions of silver nitrate and an alkali hydroxide. This reaction does not afford appreciable amounts of silver hydroxide due to the favorable energetics for the following reaction:
US patent 20050050990 describes the preparation of Ag2O with properties suitable for use as a fine grained conductive paste filler.
Ag2O features linear, two-coordinate Ag centers linked by tetrahedral oxides. It is isostructural with Cu2O. It "dissolves" in solvents that degrade it. It is slightly soluble in water due to the formation of the ion Ag(OH)2− and possibly related hydrolysis products. It dissolves in ammonia solution to give soluble derivatives. A slurry of Ag2O is readily attacked by acids:
where HX = HF, HCl, HBr, or HI, HO2CCF3. It will also react with solutions of alkali chlorides to precipitate silver chloride, leaving a solution of the corresponding alkali hydroxide.
Like many silver compounds, silver oxide is photosensitive. It also decomposes at temperatures above 280 °C.
This oxide is used in some silver-oxide batteries, as is the silver(I,III)oxide, Ag4O4. In organic chemistry, silver oxide is used as a mild oxidizing agent. For example, it oxidizes aldehydes to carboxylic acids. Such reactions often work best when the silver oxide is prepared in situ from silver nitrate and alkali hydroxide.
...
Wikipedia

