Zirconium(IV) chloride
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC names
Zirconium tetrachloride
Zirconium(IV) chloride |
|
| Identifiers | |
|
10026-11-6 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:77566 |
| ChemSpider |
23202 |
| ECHA InfoCard | 100.030.041 |
| EC Number | 233-058-2 |
| PubChem | 24817 |
| UNII |
Z88176T871 |
|
|
|
|
| Properties | |
| ZrCl4 | |
| Molar mass | 233.04 g/mol |
| Appearance | white crystals hygroscopic |
| Density | 2.80 g/cm3 |
| Melting point | 437 °C (819 °F; 710 K) (triple point) |
| Boiling point | 331 °C (628 °F; 604 K) (sublimes) |
| hydrolysis | |
| Solubility | soluble in alcohol, ether, concentrated HCl |
| Structure | |
| Monoclinic, mP10 | |
| P12/c1, No. 13 | |
| Thermochemistry | |
| 125.38 J K−1 mol−1 | |
|
Std molar
entropy (S |
181.41 J K−1 mol−1 |
|
Std enthalpy of
formation (ΔfH |
−980.52 kJ/mol |
| Hazards | |
| Safety data sheet | MSDS |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
1688 mg/kg (oral, rat) 655 mg/kg (mouse, oral) |
| Related compounds | |
|
Other anions
|
Zirconium(IV) fluoride Zirconium(IV) bromide Zirconium(IV) iodide |
|
Other cations
|
Titanium tetrachloride Hafnium tetrachloride |
|
Related compounds
|
Zirconium(II) chloride, Zirconium(III) chloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Zirconium(IV) chloride, also known as zirconium tetrachloride, (ZrCl4) is an inorganic compound frequently used as a precursor to other compounds of zirconium. This white high-melting solid hydrolyzes rapidly in humid air.
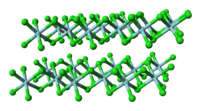
Unlike molecular TiCl4, solid ZrCl4 adopts a polymeric structure wherein each Zr is octahedrally coordinated. This difference in structures is responsible for the striking difference in their properties: TiCl
4 is distillable, but ZrCl
4 is a solid with a high melting point. In the solid state, ZrCl4 adopts a tape-like linear polymeric structure—the same structure adopted by HfCl4. This polymer degrades readily upon treatment with Lewis bases, which cleave the Zr-Cl-Zr linkages.
This conversion entails treatment of the oxide with carbon as the oxide "getter" and chlorine.
A laboratory scale process uses carbon tetrachloride in place of carbon and chlorine:
ZrCl4 is an intermediate in the conversion of zirconium minerals to metallic zirconium by the Kroll process. In nature, zirconium minerals invariably exist as oxides (reflected also by the tendency of all zirconium chlorides to hydrolyze). For their conversion to bulk metal, these refractory oxides are first converted to the tetrachloride, which can be distilled at high temperatures. The purified ZrCl4 can be reduced with Zr metal to produce zirconium(III) chloride.
ZrCl4 is the most common precursor for chemical vapor deposition of zirconium dioxide and zirconium diboride.
...
Wikipedia

