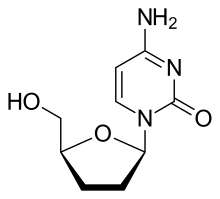
Zalcitabine
 |
|
| Clinical data | |
|---|---|
| Trade names | Hivid (discontinued) |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | >80% |
| Protein binding | <4% |
| Metabolism | Hepatic |
| Biological half-life | 2 hours |
| Excretion | Renal (circa 80%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| ECHA InfoCard | 100.149.677 |
| Chemical and physical data | |
| Formula | C9H13N3O3 |
| Molar mass | 211.218 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Zalcitabine (2′-3′-dideoxycytidine, ddC), also called dideoxycytidine, is a nucleoside analog reverse transcriptase inhibitor (NRTI) sold under the trade name Hivid. Zalcitabine was the third antiretroviral to be approved by the Food and Drug Administration (FDA) for the treatment of HIV/AIDS. It is used as part of a combination regimen.
Zalcitabine appears less potent than some other nucleoside RTIs, has an inconvenient three-times daily frequency and is associated with serious adverse events. For these reasons it is now rarely used to treat human immunodeficiency virus (HIV), and it has even been removed from pharmacies entirely in some countries.
Zalcitabine was first synthesized in the 1960s by Jerome Horwitz and subsequently developed as an anti-HIV agent by Samuel Broder, Hiroaki Mitsuya, and Robert Yarchoan at the National Cancer Institute (NCI). Like didanosine, it was then licensed because the NCI may not market or sell drugs. The National Institutes of Health (NIH) thus licensed it to Hoffmann-La Roche.
Zalcitabine was the third antiretroviral to be approved by the Food and Drug Administration (FDA) for the treatment of HIV/AIDS. It was approved on June 19, 1992 as a monotherapy and again in 1996 for use in combination with zidovudine (AZT). Using combinations of NRTIs was in practice prior to the second FDA approval and the triple drug combinations with dual NRTIs and a protease inhibitor (PI) were not far off by this time.
The sale and distribution of zalcitabine has been discontinued since December 31, 2006.
Zalcitabine is an analog of pyrimidine. It is a derivative of the naturally existing deoxycytidine, made by replacing the hydroxyl group in position 3' with a hydrogen.
...
Wikipedia
