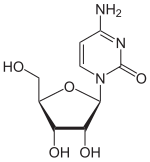
Cytidine
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
4-amino-1-[3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2-one
|
|
| Identifiers | |
|
65-46-3 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:17562 |
| ChEMBL |
ChEMBL95606 |
| ChemSpider |
5940 |
| ECHA InfoCard | 100.000.555 |
| 4728 | |
| KEGG |
D07769 |
| MeSH | Cytidine |
| PubChem | 6175 |
| UNII |
5CSZ8459RP |
|
|
|
|
| Properties | |
| C9H13N3O5 | |
| Molar mass | 243.217 |
| -123.7·10−6 cm3/mol | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Cytidine is a nucleoside molecule that is formed when cytosine is attached to a ribose ring (also known as a ribofuranose) via a β-N1-glycosidic bond. Cytidine is a component of RNA.
If cytosine is attached to a deoxyribose ring, it is known as a deoxycytidine.
Dietary sources of cytidine include foods with high RNA (ribonucleic acid) content, such as organ meats, Brewer's yeast, as well as pyrimidine-rich foods such as beer. During digestion, RNA-rich foods are broken-down into ribosyl pyrimidines (cytidine and uridine), which are absorbed intact. In humans, dietary cytidine is converted into uridine, which is probably the compound behind cytidine's metabolic effects.
There are a variety of cytidine analogues with potentially useful pharmacology. For example, KP-1461 is an anti-HIV agent that works as a viral mutagen, and zebularine exists in E. coli and is being examined for chemotherapy. Low doses of azacitidine and its analog decitabine have shown results against cancer through epigenetic demethylation.
In addition to its role as a pyrimidine component of RNA, cytidine has been found to control neuronal-glial glutamate cycling, with supplementation decreasing midfrontal/cerebral glutamate/glutamine levels. As such, cytidine has generated interest as a potential glutamatergic antidepressant drug.
...
Wikipedia
