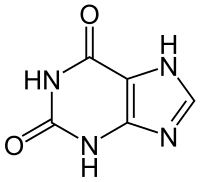
Xanthine
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
3,7-Dihydropurine-2,6-dione
|
|
| Other names
1H-Purine-2,6-dione
|
|
| Identifiers | |
|
69-89-6 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:17712 |
| ChEMBL |
ChEMBL1424 |
| ChemSpider |
1151 |
| DrugBank |
DB02134 |
| ECHA InfoCard | 100.000.653 |
| 4557 | |
| KEGG |
C00385 |
| PubChem | 1188 |
| UNII |
1AVZ07U9S7 |
|
|
|
|
| Properties | |
| C5H4N4O2 | |
| Molar mass | 152.11 g/mol |
| Appearance | White solid |
| Melting point | decomposes |
| 1 g/ 14.5 L @ 16 °C 1 g/1.4 L @ 100 °C |
|
| Hazards | |
| NFPA 704 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Xanthine (/ˈzænθiːn/ or /ˈzænθaɪn/; archaically xanthic acid) (3,7-dihydropurine-2,6-dione), is a purine base found in most human body tissues and fluids and in other organisms. A number of stimulants are derived from xanthine, including caffeine and theobromine.
Xanthine is a product on the pathway of purine degradation.
Xanthine is subsequently converted to uric acid by the action of the xanthine oxidase enzyme.
Studies reported in 2008, based on 12C/13C isotopic ratios of organic compounds found in the Murchison meteorite, suggested that xanthine and related chemicals, including the RNA component uracil, were formed extraterrestrially. In August 2011, a report, based on NASA studies with meteorites found on Earth, was published suggesting xanthine and related organic molecules, including the DNA and RNA components adenine and guanine, were found in outer space.
...
Wikipedia

