Theobromine
 |
|
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic demethylation and oxidation |
| Biological half-life | 7.1±0.7 hours |
| Excretion | Renal (10% unchanged, rest as metabolites) |
| Identifiers | |
|
|
| Synonyms | xantheose diurobromine 3,7-dimethylxanthine |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.001.359 |
| Chemical and physical data | |
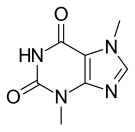
| Formula | C7H8N4O2 |
| Molar mass | 180.164 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Theobromine, formerly known as xantheose, is a bitter alkaloid of the cacao plant, with the chemical formula C7H8N4O2. It is found in chocolate, as well as in a number of other foods, including the leaves of the tea plant, and the kola (or cola) nut. It is classified as a xanthine alkaloid, which also include the similar compounds theophylline and caffeine. The compounds differ in that caffeine has an extra methyl group (see under #Pharmacology).
Despite its name, the compound contains no bromine—theobromine is derived from Theobroma, the name of the genus of the cacao tree, (which itself is made up of the Greek roots theo ("god") and broma ("food"), meaning "food of the gods" with the suffix -ine given to alkaloids and other basic nitrogen-containing compounds.
Theobromine is a slightly water-soluble (330 mg/L), crystalline, bitter powder. Theobromine is white or colourless, but commercial samples can be yellowish. It has an effect similar to, but lesser than, that of caffeine in the human nervous system, making it a lesser homologue. Theobromine is an isomer of theophylline, as well as paraxanthine. Theobromine is categorized as a dimethyl xanthine.
...
Wikipedia
