Titanium(IV) fluoride
 |
|
| Names | |
|---|---|
| Other names
titanium tetrafluoride
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.106 |
| EC Number | 232-017-6 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| TiF4 | |
| Molar mass | 123.861 g/mol |
| Appearance | white powder hygroscopic |
| Density | 2.798 g/cm3 |
| Melting point | 377 °C (711 °F; 650 K) |
| Boiling point | sublimes |
| Hazards | |
|
EU classification (DSD) (outdated)
|
not listed |
| NFPA 704 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
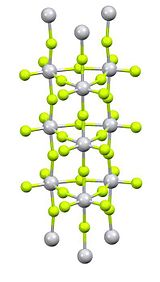
Titanium(IV) fluoride is the inorganic compound with the formula TiF4. It is a white hygroscopic solid. In contrast to the other tetrahalides of titanium, it adopts a polymeric structure. In common with the other tetrahalides, TiF4 is a strong Lewis acid.
The traditional method involves treatment of titanium tetrachloride with excess hydrogen fluoride:
Purification is by sublimation, which involves reversible cracking of the polymeric structure. X-ray crystallography reveals that the Ti centres are octahedral, but conjoined in an unusual columnar structure.
TiF4 forms adducts with many ligands. One example is cis-TiF4(MeCN)2, which is formed by treatment with acetonitrile.
...
Wikipedia

