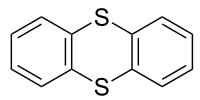
Thianthrene
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Thianthrene
|
|
| Other names
Thianthren; 9,10-Dithiaanthracene; Di-o-phenylene disulfide
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.001.998 |
| EC Number | 202-197-0 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C12H8S2 | |
| Molar mass | 216.32 g·mol−1 |
| Melting point | 151 to 155 °C (304 to 311 °F; 424 to 428 K) |
| Boiling point | 364 to 366 °C (687 to 691 °F; 637 to 639 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Thianthrene is a sulfur-containing heterocyclic chemical compound. It is a derivative of the parent heterocycle called dithiin. It is notable for its ease of oxidation.
Like other 1,4-dithiins but unlike its oxygen analog dibenzodioxin, the shape of thianthrene is not planar. It is bent, with a fold angle of 128° between the two benzo groups.
Thianthrene can be prepared by treating benzene with disulfur dichloride in the presence of aluminium chloride.
Thianthrene was first synthesized by John Stenhouse by dry distillation of sodium benzenesulfonate. Thianthrene is oxidized by sulfuric acid forming a red radical cation. Thianthrene•+ has been characterized by Electron paramagnetic resonance. Four different publications describe the crystal structure of salts of thianthrene•+.
...
Wikipedia
