Suvorexant
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Belsomra |
| AHFS/Drugs.com | belsomra |
| MedlinePlus | a614046 |
| Pregnancy category |
|
| Dependence liability |
Moderate |
| Routes of administration |
By mouth |
| ATC code | None |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 82% (at 10 mg) |
| Protein binding | >99% |
| Metabolism | hepatic, CYP3A, CYP2C19 |
| Biological half-life | ~12 hours |
| Excretion | Feces (66%), urine (23%) |
| Identifiers | |
|
|
| Synonyms | MK-4305 |
| CAS Number |
1030377-33-3 |
| PubChem (CID) | 24965990 |
| IUPHAR/BPS | 2890 |
| ChemSpider |
4589156 |
| UNII |
081L192FO9 |
| ChEMBL |
CHEMBL1083659 |
| ECHA InfoCard | 100.210.546 |
| Chemical and physical data | |
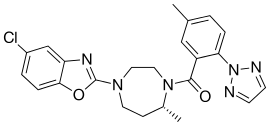
| Formula | C23H23ClN6O2 |
| Molar mass | 450.92 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
|
|
|
Suvorexant, sold under the trade name Belsomra, is a medication for the treatment of insomnia. It is effective for insomnia, at least for four weeks and as compared to a placebo.
Suvorexant is a selective, dual orexin receptor antagonist made by Merck & Co. It was approved for sale by the U.S. Food and Drug Administration (FDA) on August 13, 2014. The U.S. Drug Enforcement Administration (DEA) has placed it on the list of schedule IV controlled substances, as it may lead to limited physical dependence or psychological dependence relative to the drugs or other substances in schedule III. The potential for psychological dependence is similar to that of zolpidem. The drug became available in Japan in November 2014 and in the United States in February 2015.
Suvorexant is used for the treatment of insomnia, characterized by difficulties with sleep onset and/or sleep maintenance.
It is unclear how this medication compares to others used for insomnia as no comparisons have been done. It is also unclear if this medication is safe among people with a history of addiction, as they were excluded from the clinical trials of suvorexant.
The most common complaint about the drug is from users who report that it did not help them to sleep. Some people reported that the drug caused a sleep disturbance such as a nightmare, sleep terror, or abnormal dream. Others reported that the drug caused them to be more awake.
Issues include sleepiness the next day and issues with driving. Other concerns include thoughts of suicide.
...
Wikipedia
