Sucralose
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
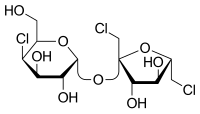
(1→6)-Dichloro-(1→6)-dideoxy-β-D-fructofuranosyl-4-chloro-4-deoxy-α-D-galactopyranoside
|
|
|
Systematic IUPAC name
(2R,3R,4R,5R,6R)-2-[(2R,3S,4S,5S)-2,5-Bis(chloromethyl)-3,4-dihydroxyoxolan-2-yl]oxy-5-chloro-6-(hydroxymethyl)oxane-3,4-diol
|
|
| Other names
1',4,6'-Trichlorogalactosucrose; Trichlorosucrose; E955; 4,1',6'-Trichloro-4,1',6'-trideoxygalactosucrose; TGS; Splenda
|
|
| Identifiers | |
|
56038-13-2 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:32159 |
| ChemSpider |
64561 |
| ECHA InfoCard | 100.054.484 |
| EC Number | 259-952-2 |
| E number | E955 (glazing agents, ...) |
| KEGG |
C12285 |
| PubChem | 71485 |
| UNII |
96K6UQ3ZD4 |
|
|
|
|
| Properties | |
| C12H19Cl3O8 | |
| Molar mass | 397.64 g/mol |
| Appearance | Off-white to white powder |
| Odor | Odorless |
| Density | 1.69 g/cm3 |
| Melting point | 125 °C (257 °F; 398 K) |
| 283 g/L (20°C) | |
| Acidity (pKa) | 12.52±0.70 |
| Hazards | |
| NFPA 704 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Sucralose is an artificial sweetener and sugar substitute. The majority of ingested sucralose is not broken down by the body, so it is noncaloric. In the European Union, it is also known under the E number E955. Sucralose is about 320 to 1,000 times sweeter than sucrose, three times as sweet as aspartame, twice as sweet as saccharin and three times as sweet as acesulfame potassium. It is stable under heat and over a broad range of pH conditions. Therefore, it can be used in baking or in products that require a longer shelf life. The commercial success of sucralose-based products stems from its favorable comparison to other low-calorie sweeteners in terms of taste, stability, and safety. Common brand names of sucralose-based sweeteners are Splenda, Zerocal, Sukrana, SucraPlus, Candys, Cukren, and Nevella. Canderel Yellow also contains Sucralose, but the original Canderel and Green Canderel do not.
Sucralose was discovered in 1976 by scientists from Tate & Lyle, working with researchers Leslie Hough and Shashikant Phadnis at Queen Elizabeth College (now part of King's College London). While researching ways to use sucrose and its synthetic derivatives for industrial use, Phadnis was told to "test" a chlorinated sugar compound. Phadnis thought Hough asked him to "taste" it, so he did. He found the compound to be exceptionally sweet.
Tate & Lyle patented the substance in 1976; as of 2008, the only remaining patents concern specific manufacturing processes.
Sucralose was first approved for use in Canada in 1991. Subsequent approvals came in Australia in 1993, in New Zealand in 1996, in the United States in 1998, and in the European Union in 2004. By 2008, it had been approved in over 80 countries, including Mexico, Brazil, China, India, and Japan. In 2006, the US Food and Drug Administration amended the regulations for foods to include sucralose as a "non-nutritive sweetener" in food. In May 2008, Fusion Nutraceuticals launched a generic product to the market, using Tate & Lyle patents.
...
Wikipedia

