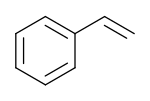
Styrene
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
Ethenylbenzene
|
|
| Other names
Styrene
Vinylbenzene Phenylethene Phenylethylene Cinnamene Styrol Diarex HF 77 Styrolene Styropol |
|
| Identifiers | |
|
100-42-5 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:27452 |
| ChEMBL |
ChEMBL285235 |
| ChemSpider |
7220 |
| ECHA InfoCard | 100.002.592 |
| KEGG |
C07083 |
| PubChem | 7501 |
| RTECS number | WL3675000 |
| UNII |
44LJ2U959V |
|
|
|
|
| Properties | |
| C8H8 | |
| Molar mass | 104.15 g/mol |
| Appearance | colorless oily liquid |
| Odor | sweet, floral |
| Density | 0.909 g/cm3 |
| Melting point | −30 °C (−22 °F; 243 K) |
| Boiling point | 145 °C (293 °F; 418 K) |
| 0.03% (20°C) | |
| Vapor pressure | 5 mmHg (20°C) |
| -68.2·10−6 cm3/mol | |
|
Refractive index (nD)
|
1.5469 |
| Viscosity | 0.762 cP at 20 °C |
| Structure | |
| 0.13 D | |
| Hazards | |
| Main hazards | flammable, toxic |
| Safety data sheet | MSDS |
| R-phrases | R10 R36 |
| S-phrases | S38 S20 S23 |
| NFPA 704 | |
| Flash point | 31 °C (88 °F; 304 K) |
| Explosive limits | 0.9%-6.8% |
| Lethal dose or concentration (LD, LC): | |
|
LC50 (median concentration)
|
2194 ppm (mouse, 4 hr) 5543 ppm (rat, 4 hr) |
|
LCLo (lowest published)
|
10,000 ppm (human, 30 min) 2771 ppm (rat, 4 hr) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 100 ppm C 200 ppm 600 ppm (5-minute maximum peak in any 3 hours) |
|
REL (Recommended)
|
TWA 50 ppm (215 mg/m3) ST 100 ppm (425 mg/m3) |
|
IDLH (Immediate danger)
|
700 ppm |
| Related compounds | |
|
Related styrenes;
related aromatic compounds |
Polystyrene, Stilbene; Ethylbenzene |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Styrene, also known as ethenylbenzene, vinylbenzene, and phenylethene, is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid that evaporates easily and has a sweet smell, although high concentrations have a less pleasant odor. Styrene is the precursor to polystyrene and several copolymers. Approximately 25 million tonnes (55 billion pounds) of styrene were produced in 2010.
Styrene is named for storax balsam, the resin of Liquidambar trees of the Altingiaceae plant family. Styrene occurs naturally in small quantities in some plants and foods (cinnamon, coffee beans, and peanuts), and is also found in coal tar.
In 1839, the German apothecary Eduard Simon isolated a volatile oil from the resin (called storax or styrax (Latin)) of the American sweetgum tree (Liquidambar styraciflua). He called the oil "Styrol" (now: "styrene"). He also noticed that when Styrol was exposed to air, light, or heat, it gradually transformed into a hard, rubber-like substance, which he called "Styroloxyd" (styrol oxide, now: "polystyrene"). By 1845, the German chemist August Hofmann and his student John Blyth (1814–1871) had determined Styrol's empirical formula: C8H8. They had also determined that Simon's "Styroloxyd" — which they renamed "Metastyrol" — had the same empirical formula as Styrol. Furthermore, they could obtain Styrol by dry distilling Metastyrol. In 1865, the German chemist Emil Erlenmeyer found that Styrol could form a dimer, and in 1866 the French chemist Marcelin Berthelot stated that Metastyrol was a polymer of Styrol. Meanwhile, other chemists had been investigating another component of storax, namely, cinnamic acid. They had found that cinnamic acid could be decarboxylated to form cinnamène (or cinnamol), which appeared to be Styrol. In 1845, French chemist Emil Kopp suggested that the two compounds were identical, and in 1866, Erlenmeyer suggested that both cinnamol and Styrol might be vinyl benzene. However, the Styrol that was obtained from cinnamic acid seemed different from the Styrol that was obtained by distilling storax resin: the latter was optically active. Eventually, in 1876, the Dutch chemist van 't Hoff resolved the ambiguity: the optical activity of the Styrol that was obtained by distilling storax resin was due to a contaminant.
...
Wikipedia

