Stichodactyla toxin
| ShK domain-like | |||||||||
|---|---|---|---|---|---|---|---|---|---|
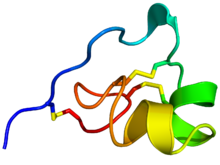
Rainbow colored cartoon diagram (N-terminus = blue, C-terminus = red) of an NMR solution structure of the ShK toxin. Sidechains of cysteine residues involved in disulfide linkages are displayed as sticks and the sulfur atoms in these links are colored yellow.
|
|||||||||
| Identifiers | |||||||||
| Symbol | ShK | ||||||||
| Pfam | PF01549 | ||||||||
| InterPro | IPR003582 | ||||||||
| SMART | SM00254 | ||||||||
| SCOP | 1roo | ||||||||
| SUPERFAMILY | 1roo | ||||||||
| TCDB | 8.B.14 | ||||||||
| OPM superfamily | 475 | ||||||||
| OPM protein | 2lg4 | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
Stichodactyla toxin, also called ShK toxin, is a peptide toxin that blocks the voltage-gated potassium channels: Kv1.1, Kv1.3, Kv1.6, Kv3.2 and KCa3.1.
ShK is a 35-residue basic peptide first discovered in the sea anemone Stichodactyla helianthus by Olga Castaneda from the University of Havana, Cuba, and her collaborators in Sweden. The formula is C169H274N54O48S7. It is cross-linked by three disulfide bridges: Cys3-Cys35, Cys12-Cys28, and Cys17-Cys32 (see figure below). The amino acid sequence of the ShK toxin is Arg-Ser-Cys-Ile-Asp-Thr-Ile-Pro-Lys-Ser-Arg-Cys-Thr-Ala-Phe-Gln-Cys-Lys-His-Ser-Met-Lys-Tyr-Arg-Leu-Ser-Phe-Cys-Arg-Lys-Thr-Cys-Gly-Thr-Cys. ShK is stabilized by three disulfide bridges and consists of two short α-helices comprising residues 14-19 and 21-24. The N-terminal eight residues of ShK adopt an extended conformation, followed by a pair of interlocking turns that resemble a 310 helix, while its C-terminal Cys35 residue forms a nearly head-to-tail cyclic structure through a disulfide bond with Cys3.Protein domains with structural resemblance to ShK have been described in 688 proteins, most of them from Caenorhabditis elegans (InterPro: IPR003582). The SMART database at the EMBL has a list of 688 proteins containing 1315 ShK-like sequences (http://smart.embl-heidelberg.de). Other proteins containing domains with similar structures include the cysteine-rich secretory protein snake toxins natrin, triflin, and stecrisp, the Toxocara canis mucins, secreted peptides from the dog hookworm Ancylostoma caninum, and the human proteins Tpx-1 and matrix metalloprotease 23 (MMP23).
...
Wikipedia
