Sodium Bicarbonate
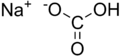 |
|||
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Sodium hydrogen carbonate
|
|||
| Other names
Baking soda, bicarb (laboratory slang), bicarbonate of soda, nahcolite
|
|||
| Identifiers | |||
|
144-55-8 |
|||
| 3D model (Jmol) | Interactive image | ||
| 4153970 | |||
| ChEBI |
CHEBI:32139 |
||
| ChEMBL |
ChEMBL1353 |
||
| ChemSpider |
8609 |
||
| DrugBank |
DB01390 |
||
| ECHA InfoCard | 100.005.122 | ||
| EC Number | 205-633-8 | ||
| 4507 | |||
| KEGG |
C12603 |
||
| MeSH | Sodium+bicarbonate | ||
| PubChem | 516892 | ||
| RTECS number | VZ0950000 | ||
| UNII |
8MDF5V39QO |
||
|
|||
|
|||
| Properties | |||
| NaHCO 3 |
|||
| Molar mass | 84.0066 g mol−1 | ||
| Appearance | White crystals | ||
| Odor | odorless | ||
| Density | 2.20 g/cm3 as a solid 1.1 to 1.3 as a powder |
||
| Melting point | (decomposes to sodium carbonate starting at 50 °C) | ||
| 69 g/L (0 °C) 96 g/L (20 °C) |
|||
| Solubility | 0.02 wt% acetone, 2.13 wt% methanol @22 °C. insoluble in ethanol | ||
| log P | -0.82 | ||
| Acidity (pKa) | 10.329 6.351 (carbonic acid) |
||
|
Refractive index (nD)
|
nα = 1.377 nβ = 1.501 nγ = 1.583 | ||
| Structure | |||
| monoclinic | |||
| Thermochemistry | |||
| 87.61 J/mol K | |||
|
Std molar
entropy (S |
102 J/mol K | ||
|
Std enthalpy of
formation (ΔfH |
-947.7 kJ/mol | ||
|
Gibbs free energy (ΔfG˚)
|
-851.9 kJ/mol | ||
| Pharmacology | |||
| B05CB04 (WHO) B05XA02 (WHO), QG04BQ01 (WHO) | |||
| Intravenous, oral | |||
| Hazards | |||
| Main hazards | Causes serious eye irritation | ||
| Safety data sheet | External MSDS | ||
| NFPA 704 | |||
| Flash point | incombustible | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
4220 mg/kg ( rat, oral ) | ||
| Related compounds | |||
|
Other anions
|
Sodium carbonate | ||
|
Other cations
|
Ammonium bicarbonate |
||
|
Related compounds
|
Sodium bisulfate |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
1.1 to 1.3 as a powder
96 g/L (20 °C)
165 g/L (60 °C)
236 g/L (100 °C)
6.351 (carbonic acid)
Sodium bicarbonate (IUPAC name: sodium hydrogen carbonate) is a chemical compound with the formula NaHCO3. It is a salt composed of sodium ions and bicarbonate ions. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda (sodium carbonate). The natural mineral form is nahcolite. It is a component of the mineral natron and is found dissolved in many mineral springs. It is among the food additives encoded by the European Union, identified as E 500. Since it has long been known and is widely used, the salt has many related names such as baking soda, bread soda, cooking soda, and bicarbonate of soda. In colloquial usage, the names sodium bicarbonate and bicarbonate of soda are often truncated. Forms such as sodium bicarb, bicarb soda, bicarbonate, bicarb, or even bica are common. The word saleratus, from Latin sal æratus meaning "aerated salt", was widely used in the 19th century for both sodium bicarbonate and potassium bicarbonate.
The prefix "bi" in "bicarbonate" comes from an outdated naming system and is based on the observation that there is twice as much carbonate (CO3) per sodium in sodium bicarbonate (NaHCO3) as there is carbonate per sodium in sodium carbonate (Na2CO3) and other carbonates. The modern way of analyzing the situation based on the exact chemical composition (which was unknown when the name "sodium bicarbonate" was coined) says this the other way around: there is half as much sodium in NaHCO3 as in Na2CO3 (Na versus Na2).
...
Wikipedia



