Selenous acid
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Selenous acid
|
|
| Identifiers | |
|
7783-00-8 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:26642 |
| ChemSpider |
1060 |
| ECHA InfoCard | 100.029.067 |
| KEGG |
D05814 |
| PubChem | 1091 |
| UNII |
F6A27P4Q4R |
|
|
|
|
| Properties | |
| H2SeO3 | |
| Molar mass | 128.97 g/mol |
| Appearance | white hygroscopic crystals |
| Density | 3.0 g/cm3 |
| Melting point | decomposes at 70°C |
| very soluble | |
| Solubility | soluble in ethanol |
| Acidity (pKa) | 2.46, 7.3 |
| −45.4·10−6 cm3/mol | |
| Related compounds | |
|
Other anions
|
selenic acid hydrogen selenide |
|
Other cations
|
sodium selenite |
|
Related compounds
|
sulfurous acid tellurous acid polonous acid |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
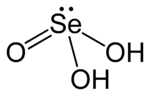
Selenous acid (or selenious acid) is the chemical compound with the formula H2SeO3. Structurally, it is more accurately described by (HO)2SeO. It is the principal oxoacid of selenium; the other being selenic acid.
Selenous acid is analogous to sulfurous acid, but it is more readily isolated. Selenous acid is easily formed upon the addition of selenium dioxide to water. As a crystalline solid, the compound can be seen as pyramidal molecules that are interconnected with hydrogen bonds. In solution it is a diprotic acid:
It is moderately oxidizing in nature, but kinetically slow. In 1 M H+
:
In 1 M OH−
:
It is used in organic synthesis for the synthesis of 1,2-diketones (e.g. glyoxal).
The major use is in protecting and changing the color of steel, especially steel parts on firearms. The so-called cold-bluing process uses selenous acid, copper(II) nitrate, and nitric acid to change the color of the steel from silver-grey to blue-grey or black. Alternative procedures use copper sulfate and phosphoric acid instead. This process deposits a coating of copper selenide and is fundamentally different from other bluing processes which generate black iron oxide. Some older razor blades were also made of blued steel.
...
Wikipedia
