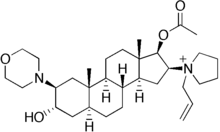
Rocuronium
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Routes of administration |
Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Protein binding | ~30% |
| Metabolism | some de-acetylation |
| Biological half-life | 66–80 minutes |
| Excretion | Unchanged, in bile and urine |
| Identifiers | |
|
|
| Synonyms | [3-hydroxy-10,13-dimethyl-2-morpholin-4-yl-16-(1-prop-2-enyl-2,3,4,5-tetrahydropyrrol-1-yl)-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl] acetate |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C32H53N2O4+ |
| Molar mass | 529.774 g/mol |
|
|
|
Rocuronium (Zemuron, Esmeron) is an aminosteroid non-depolarizing neuromuscular blocker or muscle relaxant used in modern anaesthesia to facilitate endotracheal intubation by providing skeletal muscle relaxation, most commonly required for surgery or mechanical ventilation. It is used for both standard endotracheal intubation and rapid sequence induction (RSI), although Suxamethonium chloride is usually selected for RSI given its faster onset of action compared to rocuronium.
It was designed to be a weaker antagonist at the neuromuscular junction than pancuronium; hence its monoquaternary structure and its having an allyl group and a pyrrolidine group attached to the D ring quaternary nitrogen atom. Rocuronium has a rapid onset and intermediate duration of action.
There is considered to be a risk of allergic reaction to the drug in some patients (particularly those with asthma), but a similar incidence of allergic reactions has been observed by using other members of the same drug class (non-depolarizing neuromuscular blocking drugs).
The γ-cyclodextrin derivative sugammadex (trade name Bridion) has been recently introduced as a novel agent to reverse the action of rocuronium.Sugammadex has been in use since 2009 in many European countries; however, it was turned down for approval twice by the US FDA due to concerns over allergic reactions and bleeding, but finally approved the medication for use during surgical procedures in the United States on December 15, 2015.Neostigmine can also be used as a reversal agent of rocuronium but is not as effective as sugammadex. Neostigmine is often still used due to its lower cost compared to sugammadex.
...
Wikipedia
